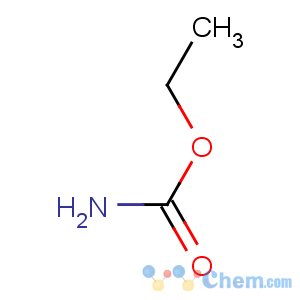
Title: Urethan
CAS Registry Number: 51-79-6
CAS Name: Carbamic acid ethyl ester
Synonyms: ethyl carbamate; urethane; ethyl urethan
Molecular Formula: C3H7NO2
Molecular Weight: 89.09
Percent Composition: C 40.44%, H 7.92%, N 15.72%, O 35.92%
Line Formula: NH2COOC2H5
Literature References: Naturally occurring contaminant in fermented foods, particularly wine, stone-fruit brandies, and bread. Prepd by heating urea with alcohol under pressure; by warming urea nitrate with alcohol and sodium nitrite. Toxicity data: K. J. Franklin,
J. Pharmacol. Exp. Ther. 42, 1 (1931). Review of carcinogenic action and metabolism: S. S. Mirvish in
Adv. Cancer Res. 11, 1-42 (1968); of mutagenicity, metabolism and interactions with DNA: R. E. Sotomayor, T. F. X. Collins,
Toxicol. Ind. Health 6, 71-108 (1990); of physiological effects in animals: K. J. Field, C. M. Lang,
Lab. Animals 22, 255-262 (1988). Review of analysis, occurrence and formation in foodstuffs: R. Battaglia
et al., Food Addit. Contam. 7, 477-496 (1990); B. Zimmerli, J. Schlatter,
Mutat. Res. 259, 325-350 (1991).
Properties: Crystals, mp 48-50°. Cooling saline taste, d 1.1. bp 182-184°. Sublimes readily at 103° and 54 mm pressure. One gram dissolves in 0.5 ml water, 0.8 ml alcohol, 0.9 ml chloroform, 1.5 ml ether, 2.5 ml glycerol, 32 ml olive oil. The aq soln is neutral. MLD i.p. in mice: 2.1-2.2 g/kg (Franklin).
Melting point: mp 48-50°
Boiling point: bp 182-184°
Density: d 1.1
Toxicity data: MLD i.p. in mice: 2.1-2.2 g/kg (Franklin)
CAUTION: This substance is reasonably anticipated to be a human carcinogen:
Report on Carcinogens, Eleventh Edition (PB2005-104914, 2004) p III-270.
Use: Intermediate in organic synthesis. In the prepn and modification of amino resins. As solvent, solubilizer and cosolvent for various organic materials. Animal anesthetic in laboratory procedures.