Title: Cobaltous Carbonate
CAS Registry Number: 513-79-1
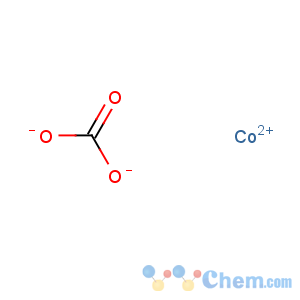
Molecular Formula: CCoO3
Molecular Weight: 118.94
Percent Composition: C 10.10%, Co 49.55%, O 40.35%
Line Formula: CoCO3
Literature References: Occurs in nature as the mineral
cobalt spar or
sphaerocobaltite. Prepd by heating a soln of a cobaltous salt with Na2CO3: Schlessinger,
Inorg. Synth. 6, 189 (1963) where it is the starting material for the prepn of trinitrotriamminecobalt.
Review: de Bie, Doyen,
Cobalt 15, 3-13;
16, 3-15 (1962).
Properties: Red powder or rhombohedral crystals. d 4.13. Almost insol in water, alcohol, methyl acetate. Does not react with cold concd HNO3 or HCl; when heated, dissolves with evolution of CO2. Oxidized by air or weak oxidizing agents to cobaltic carbonate.
Density: d 4.13
Derivative Type: Hexahydrate
Properties: Pink to violet-red cryst needles. Pptd when excess CO2 is present during prepn. On heating becomes anhydr by 140°. Stable in air.
Derivative Type: Cobaltous carbonate basic
Synonyms: Cobalt carbonate hydroxide
Molecular Formula: C2H6Co5O12
Molecular Weight: 516.73
Percent Composition: C 4.65%, H 1.17%, Co 57.03%, O 37.16%
Properties: Co5(OH)6(CO3)2. Pale-red powder, usually containing some water. Practically insol in water; sol in dilute acids and ammonia.
Use: In ceramics; manuf of Co pigments; prepn of Co compds.
Therap-Cat-Vet: Nutritional factor. Used in cobalt deficiency in ruminants.