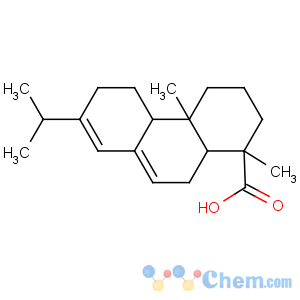
Title: Abietic Acid
CAS Registry Number: 514-10-3
CAS Name: [1
R-(1a,4ab,4ba,10aa)]-1,2,3,4,4a,4b,5,6,10,10a-Decahydro-1,4a-dimethyl-7-(1-methylethyl)-1-phenanthrenecarboxylic acid
Synonyms: 13-isopropylpodocarpa-7,13-dien-15-oic acid; sylvic acid
Molecular Formula: C20H30O2
Molecular Weight: 302.45
Percent Composition: C 79.42%, H 10.00%, O 10.58%
Literature References: A widely available organic acid, prepared by isomerization of rosin: Harris, Sanderson,
Org. Synth. coll. vol. IV, 1 (1963); Fieser, Fieser,
The Chemistry of Natural Products Related to Phenanthrene (New York, 3rd ed., 1949). Synthesis from dehydroabietic acid: A. W. Burgstahler, L. W. Worden,
J. Am. Chem. Soc. 83, 2587 (1961); E. Wenkert
et al., ibid. 86, 2038 (1964). Chromatographic study: A. G. Douglas, T. G. Powell,
J. Chromatogr. 43, 241 (1969). Metabolism in rabbits: Y. Asakawa
et al., Xenobiotica 16, 753 (1986).
Properties: Monoclinic plates from alcohol + water, mp 172-175°. [a]D24 -106° (c = 1 in abs alc). uv max: 235, 241.5, 250 nm (e 19500, 22000, 14300). Insol in water. Sol in alc, benzene, chloroform, ether, acetone, carbon disulfide, dil NaOH soln. Commercial abietic acid made by heating rosin alone or with acids may be glassy or partly crystalline, usually of yellow color and melting as low as 85°.
Melting point: mp 172-175°
Optical Rotation: [a]D24 -106° (c = 1 in abs alc)
Absorption maximum: uv max: 235, 241.5, 250 nm (e 19500, 22000, 14300)
Derivative Type: Methyl ester
see Methyl Abietate
Use: Manufacture of esters (ester gums), e.g., methyl, vinyl and glyceryl esters for use in lacquers and varnishes. Manufacture of "metal resinates", soaps, plastics and paper sizes. Assists growth of lactic and butyric acid bacteria.