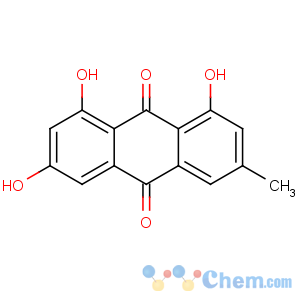
Title: Emodin
CAS Registry Number: 518-82-1
CAS Name: 1,3,8-Trihydroxy-6-methyl-9,10-anthracenedione
Synonyms: 1,3,8-trihydroxy-6-methylanthraquinone; 4,5,7-trihydroxy-2-methylanthraquinone; frangula emodin; rheum emodin; archin; frangulic acid
Molecular Formula: C15H10O5
Molecular Weight: 270.24
Percent Composition: C 66.67%, H 3.73%, O 29.60%
Literature References: Occurs mostly as the rhamnoside (
see Frangulin) in rhubarb root, in alder buckthorn
(Rhamnus frangula L.
), in
Cascara sagrada (Rhamnus purshiana DC.,
Rhamnaceae), also in
Rumex and in other
Polygonaceae. Isoln from rhubarb root: Tutin, Clewer,
J. Chem. Soc. 99, 946 (1911); Carelli, Giuliano,
Farmaco Ed. Prat. 12, 184 (1957); from bark of alder buckthorn: Bridel, Charaux,
Bull. Soc. Chim. Biol. 15, 648 (1933). Identity with archin: Chaudhry
et al., J. Sci. Ind. Res. 9B, No. 6, 142 (1950),
C.A. 44, 9396h (1950). Synthesis from 3,5-dinitrophthalic anhydride and
m-cresol: Elder, Widmer,
Helv. Chim. Acta 6, 966 (1923); from 2-methylanthraquinone: Ayyangar
et al., J. Sci. Ind. Res. 20B, 493 (1961),
C.A. 57, 8514b (1962).
Properties: Orange needles from alc or by sublimation at 12 mm. mp 256-257°. Absorption max (ethanol): 222, 252, 265, 289, 437 nm (log e 4.55, 4.26, 4.27, 4.34, 4.10). Practically insol in water; sol in alc, aq alkali hydroxide solns (cherry-red color), Na2CO3 and NH3 solns. Soly at 25° (g/100 ml of satd soln): ether 0.140; chloroform 0.071; carbon tetrachloride 0.010; carbon bisulfide 0.009; benzene 0.041.
Melting point: mp 256-257°
Absorption maximum: Absorption max (ethanol): 222, 252, 265, 289, 437 nm (log e 4.55, 4.26, 4.27, 4.34, 4.10)
Derivative Type: 3-Methyl ether
Synonyms: 1,8-Dihydroxy-3-methoxy-6-methylanthraquinone; rheochrysidin
Molecular Formula: C16H12O5
Molecular Weight: 284.26
Percent Composition: C 67.60%, H 4.26%, O 28.14%
Properties: Brick-red, monoclinic needles, mp 207°. Occurs naturally as
physcione or
parietin.
Melting point: mp 207°
Derivative Type: Trimethyl ether
Molecular Formula: C18H16O5
Molecular Weight: 312.32
Percent Composition: C 69.22%, H 5.16%, O 25.61%
Properties: Pale yellow needles, mp 225°.
Melting point: mp 225°
NOTE: See also Aloe emodin.
Therap-Cat: Cathartic.
Keywords: Laxative/Cathartic.