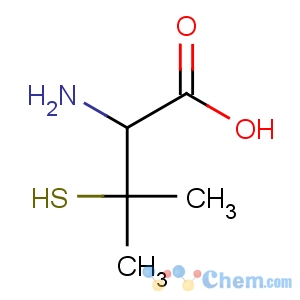
Title: Penicillamine
CAS Registry Number: 52-67-5
CAS Name: 3-Mercapto-D-valine
Synonyms: (
S)-3,3-dimethylcysteine; a-amino-b-methyl-b-mercaptobutyric acid; DMC; b-thiovaline; D-penicillamine
Trademarks: Cuprimine (Merck & Co.); Cupripen (Rubio); Depen (Wallace Labs.); D-Penamine (Dista); Distamine (Dista); Mercaptyl (Knoll); Metalcaptase (Heyl); Reumacillin (Leiras); Sufortan (Sanfer); Trisorcin (Merckle); Trolovol (Dexo)
Molecular Formula: C5H11NO2S
Molecular Weight: 149.21
Percent Composition: C 40.25%, H 7.43%, N 9.39%, O 21.45%, S 21.49%
Literature References: Characteristic degradation product of penicillin type antibiotics. Active as copper chelating agent and as a disease modifying antirheumatic drug (DMARD). Prepn by hydrolysis of penicillins: E. P. Abraham
et al., Nature 151, 107 (1943). Review of syntheses of DL-form and enantiomers: H. M. Crooks in
The Chemistry of Penicillin, H. T. Clarke
et al., Eds. (Princeton Univ. Press, 1949) pp 455-472; W. M. Weigert
et al., Angew. Chem. Int. Ed. 14, 330 (1975). Polymorphism of D-form: J. A. G. Vidler,
J. Pharm. Pharmacol. 28, 662 (1976). Toxicity data: Veis
et al., Antibiotiki 14, 837 (1969). General reviews: I. A. Jaffe in
Pharmacological and Biochemical Properties of Drug Substances Vol. 2, M. E. Goldberg, Ed. (Am. Pharm. Assoc., Washington, DC, 1979) pp 465-478; C. C. Chiu, L. T. Grady,
Anal. Profiles Drug Subs. 10, 601-637 (1981). Review of assay methods: N. Kucharczyk, S. Shahiniam,
J. Rheumatol. 8, Suppl. 7, 28-34 (1981); of metabolism and pharmacology: D. Perrett,
ibid. 41-50; of clinical pharmacokinetics: D. A. Joyce,
Pharmacol. Ther. 42, 405-427 (1989). Clinical trial in Wilson's disease: A. Czlonkowska
et al., J. Neurol. 243, 269 (1996). Review of clinical experience in rheumatoid arthritis: R. Munro, H. A. Capell,
Br. J. Rheumatol. 36, 104-109 (1997).
Properties: White or practically white, crystalline powder. mp 202-206° (Weigert). [a]D25 -63° (c = 0.1 in pyridine). Freely sol in water; slightly sol in alcohol. Insol in ether, acetone, benzene, carbon tetrachloride. LD50 in rats (mg/kg): >10000 orally, >660 i.p. (Jaffe).
Melting point: mp 202-206° (Weigert)
Optical Rotation: [a]D25 -63° (c = 0.1 in pyridine)
Toxicity data: LD50 in rats (mg/kg): >10000 orally, >660 i.p. (Jaffe)
Derivative Type: Hydrochloride
CAS Registry Number: 2219-30-9
Trademarks: Pemine (Lilly)
Molecular Formula: C5H11NO2S.HCl
Molecular Weight: 185.67
Percent Composition: C 32.34%, H 6.51%, N 7.54%, O 17.23%, S 17.27%, Cl 19.09%
Properties: Hygroscopic crystals, dec 177.5°. [a]D25 -63° (1
N NaOH). Freely sol in water, sol in ethanol. Aq solns are comparatively stable at pH 2-4. LD50 i.v. in mice: 2289 mg/kg (Veis).
Optical Rotation: [a]D25 -63° (1
N NaOH)
Toxicity data: LD50 i.v. in mice: 2289 mg/kg (Veis)
Derivative Type: DL-Form
CAS Registry Number: 52-66-4
Properties: Crystals, dec 201°. pK: 1.8 (carboxyl); 7.9 (a-amino); 10.5 (b-thiol). LD50 orally in rats: 365 mg/kg (Jaffe).
pKa: pK: 1.8 (carboxyl); 7.9 (a-amino); 10.5 (b-thiol)
Toxicity data: LD50 orally in rats: 365 mg/kg (Jaffe)
Derivative Type: DL-Form hydrochloride
CAS Registry Number: 22572-05-0
Properties: Crystals, dec 145-148°.
Derivative Type: L-Form
CAS Registry Number: 1113-41-3
Properties: Crystals, mp 190-194°. [a]D25 +63° (in 1
N NaOH). LD50 i.p. in rats: 350 mg/kg (Jaffe).
Melting point: mp 190-194°
Optical Rotation: [a]D25 +63° (in 1
N NaOH)
Toxicity data: LD50 i.p. in rats: 350 mg/kg (Jaffe)
Therap-Cat: Antirheumatic. Chelating agent (copper); Wilson's Disease treatment.
Keywords: Antiarthritic/Antirheumatic; Chelating Agent; Wilson's Disease Treatment.