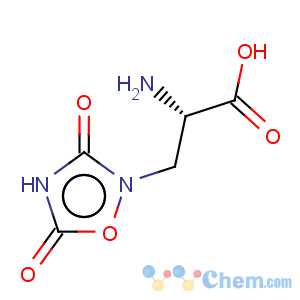
Title: Quisqualic Acid
CAS Registry Number: 52809-07-1
CAS Name: (a
S)-a-Amino-3,5-dioxo-1,2,4-oxadiazolidine-2-propanoic acid
Synonyms: L-quisqualic acid; b-(3,5-dioxo-1,2,4-oxodiazolidin-2-yl)-L-alanine
Molecular Formula: C5H7N3O5
Molecular Weight: 189.13
Percent Composition: C 31.75%, H 3.73%, N 22.22%, O 42.30%
Literature References: Excitatory amino acid (EAA) used to identify a specific subset of EAA receptors; consequently, the receptors are known as quisqualate receptors.
See also NMDA, kainic acid. Isoln from the seeds of
Quisqualis chinesis and anthelmintic activity: Y.-C. Tuan
et al., Yao Hsueh Hsueh Pao 5, 87 (1957),
C.A. 56, 14896b (1958); from
Q. indica: S.-T. Fang, J.-H. Chu,
Hua Hsueh Hsueh Pao 30, 226 (1964),
C.A. 61, 7359f (1962); from
Q. fructus: T. Takemoto
et al., Yakugaku Zasshi 95, 176 (1975),
C.A. 82, 152211a (1975). Enzymic synthesis: I. Murakoshi
et al., Chem. Pharm. Bull. 22, 473 (1974). Total synthesis: J. E. Baldwin
et al., Chem. Commun. 1985, 256. Crystal structure: J. L. Flippen, R. D. Gilardi,
Acta Crystallogr. B32, 951 (1976). Identification as a neuroexcitant: H. Shinozaki, I. Shibuya,
Neuropharmacology 13, 665 (1974). Receptor binding studies: K. Koshiya,
Life Sci. 37, 1373 (1985); J. T. Greenamyre,
J. Pharmacol. Exp. Ther. 233, 254 (1985). Review of isolation of quisqualic acid and other EAAs: T. Takemoto, in
Kainic Acid as a Tool in Neurobiology, R. G. McGeer
et al., Eds. (Raven Press, New York, 1978) pp 1-15.
Properties: Crystals from water-ethanol, mp 190-191°. [a]D20 +17.0° (c = 2.0 in 6
M HCl).
Melting point: mp 190-191°
Optical Rotation: [a]D20 +17.0° (c = 2.0 in 6
M HCl)