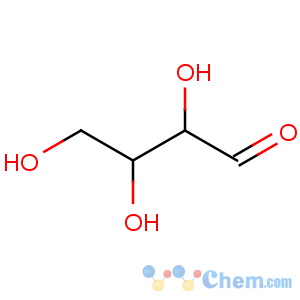
Title: L-Erythrose
CAS Registry Number: 533-49-3
CAS Name: (2
S,3
S)-2,3,4-Trihydroxybutanal
Molecular Formula: C4H8O4
Molecular Weight: 120.10
Percent Composition: C 40.00%, H 6.71%, O 53.29%
Literature References: From calcium L-arabonate by oxidation with H2O2: Ruff, Meusser,
Ber. 34, 1365 (1901). From L-arabinose oxime: Wohl,
Ber. 32, 3667 (1899). From L-arabonamide by treatment with alkaline NaOCl: Weerman,
Rec. Trav. Chim. 37, 35 (1918).
Properties: Syrup. Sweet taste. Shows mutarotation. [a]D24 +11.5° (8 min) ? +15.2° (120 min) ? +30.5° (final, c = 3): Felton, Freudenberg,
J. Am. Chem. Soc. 57, 1640 (1935). Soluble in water. Heating with HCl yields lactic acid. Oxidation with Br converts it to L-erythronic acid. Reduces Fehling's soln slowly in the cold. Not fermented by yeast.
Optical Rotation: [a]D24 +11.5° (8 min) ? +15.2° (120 min) ? +30.5° (final, c = 3): Felton, Freudenberg,
J. Am. Chem. Soc. 57, 1640 (1935)
Derivative Type: Phenylosazone
Molecular Formula: C16H18N4O2
Molecular Weight: 298.34
Percent Composition: C 64.41%, H 6.08%, N 18.78%, O 10.73%
Properties: mp 164°.
Melting point: mp 164°