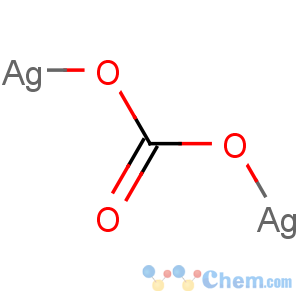
Title: Silver Carbonate
CAS Registry Number: 534-16-7
CAS Name: Carbonic acid disilver(1+) salt
Molecular Formula: CAg2O3
Molecular Weight: 275.75
Percent Composition: C 4.36%, Ag 78.24%, O 17.41%
Line Formula: Ag2CO3
Literature References: Silver carbonate pptd on Celite,
q.v., is known as
Fétizon's reagent. Thermal decompn studies: M. Centnerszwer, B. Bruzs,
J. Phys. Chem. 29, 733 (1925); P. Norby
et al., 41, 3628 (2002). Prepn from silver nitrate and sol carbonate soln: Poyer
et al., Inorg. Synth. 5, 19 (1957). Crystal structure: R. Masse
et al., Acta Crystallogr. B35, 1428 (1979). Mechanism of alcohol oxidation using silver carbonate pptd on celite: M. Fetizon, M. Golfier,
C.R. Acad. Sci. Ser. C 267, 900 (1968); M. Fetizon
et al., Tetrahedron Lett. 13, 4445 (1972); F. J. Kakis
et al., J. Org. Chem. 39, 523 (1974). Biological staining method: J. M. López-Cepero,
J. Histochem. Cytochem. 52, 211 (2004).
Properties: Light yellow powder when freshly pptd, but becomes darker on drying and on exposure to light. Dec at about 220° into Ag2O and CO2 and at higher temp into metal Ag. d 6.08. Sol in 30,000 parts cold water, 2000 parts boiling water; readily sol in dil nitric acid, ammonia, or alkali cyanides.
Protect from light.
Density: d 6.08
Use: Mild oxidizing agent for conversion of alcohols to aldehydes and ketones. Biological stain.