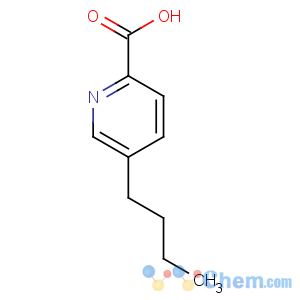
Title: Fusaric Acid
CAS Registry Number: 536-69-6
CAS Name: 5-Butyl-2-pyridinecarboxylic acid
Synonyms: 5-butylpicolinic acid
Molecular Formula: C10H13NO2
Molecular Weight: 179.22
Percent Composition: C 67.02%, H 7.31%, N 7.82%, O 17.85%
Literature References: Antibiotic (wilting agent) first isolated from the fungus
Fusarium heterosporium, Nees: Yabuta
et al., J. Agric. Chem. Soc. Jpn. 10, 1059 (1934). Isoln from other
Fusarium species and from
Gibberella fujikuroi and synthesis: Plattner
et al., Helv. Chim. Acta 37, 1379 (1954). Prepn: Hardegger, Nikles,
ibid. 39, 505 (1956);
40, 2428 (1957); Schreiber, Adam,
Ber. 93, 1848 (1960); Umezawa, Nagatsu,
DE 2005255 (1970 to Microbiochem. Res. Found.); R. Tschesche, W. Führer,
Ber. 111, 3502 (1978). Dopamine b-hydroxylase inhibitor and hypotensive activity: Suda
et al., Chem. Pharm. Bull. 17, 2377 (1969); Nagatsu
et al., Biochem. Pharmacol. 19, 35 (1970). Toxicity study: Ishii
et al., Arzneim.-Forsch. 25, 55 (1975).
Properties: Colorless crystals, mp 96-98°. LD50 orally in mice: 230 mg/kg (Ishii).
Melting point: mp 96-98°
Toxicity data: LD50 orally in mice: 230 mg/kg (Ishii)
Derivative Type: Copper salt
Properties: Bluish-violet crystals from water, mp 258-259°.
Melting point: mp 258-259°