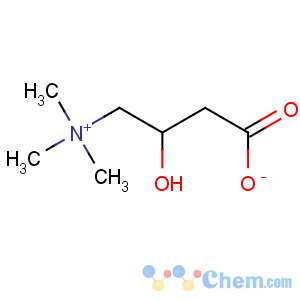
Title: Carnitine
CAS Registry Number: 541-15-1
CAS Name: (2
R)-3-Carboxy-2-hydroxy-
N,N,N-trimethyl-1-propanaminium inner salt
Synonyms: L-(3-carboxy-2-hydroxypropyl)trimethylammonium hydroxide inner salt; (-)-b-hydroxy-g-trimethylaminobutyric acid; L-carnitine; levocarnitine; vitamin BT
Trademarks: Cardiogen (UCB); Carnicor (Sigma-Tau); Carnitor (Sigma-Tau); L-Carn (Sigma-Tau); Lefcar (GSK); Levocarnil (Sigma-Tau); Miocor (Ecobi); Miotonal (Caber); Nefrocarnit (Medice)
Molecular Formula: C7H15NO3
Molecular Weight: 161.20
Percent Composition: C 52.16%, H 9.38%, N 8.69%, O 29.78%
Literature References: Essential cofactor of fatty acid metabolism; required for the transport of fatty acids through the inner mitochondrial membrane. Synthesized primarily in the liver and kidney; highest concentrations found in heart and skeletal muscle. Named derived from the Latin word,
carnis, meaning meat or flesh. Dietary sources include red meat, dairy products, beans, avocado. Isoln from meat extract: W. Gulewitsch, R. Krimberg,
Z. Physiol. Chem. 45, 326 (1905); H. E. Carter, P. K. Bhattacharyya,
Methods Enzymol. III, 660 (1957). Identification as vitamin BT: H. E. Carter
et al., Arch. Biochem. Biophys. 38, 405 (1952). Synthesis: M. Tomita, Y. Sendju,
Z. Physiol. Chem. 169, 263 (1927); R. Voeffray
et al., Helv. Chim. Acta 70, 2058 (1987). Enantioselective synthesis from glycerol: M. Marzi
et al., J. Org. Chem. 65, 6766 (2000). Biosynthesis: G. Wolf, C. R. A. Berger,
Arch. Biochem. Biophys. 92, 360 (1961). Absolute configuration: T. Kaneko, R. Yoshida,
Bull. Chem. Soc. Jpn. 35, 1153 (1962). Metabolism in humans: M. E. Mitchell,
Am. J. Clin. Nutr. 31, 293 (1978). Historical review: G. Fraenkel, S. Friedman,
Vitam. Horm. XV, 73-118 (1957). Review of physiological significance and deficiency syndromes: C. J. Rebouche, D. J. Paulson,
Annu. Rev. Nutr. 6, 41-66 (1986); of clinical pharmacology: J. J. Bahl, R. Bressler,
Annu. Rev. Pharmacol. Toxicol. 27, 257-277 (1987); of biosynthesis in mammals: F. M. Vaz, R. J. A. Wanders,
Biochem. J. 361, 417-429 (2002). Review of effect on myocardial metabolism and clinical experience in ischemic heart disease: R. Lango
et al., Cardiovasc. Res. 51, 21-29 (2001); of use as dietary supplement in athletes: H. Karlic, A. Lohninger,
Nutrition 20, 709-715 (2004). Symposium on physiology, pharmacology and therapeutic potential:
Ann. N.Y. Acad. Sci. 1033, 1-197 (2004).
Properties: Crystals from anhydrous ethanol + acetone, dec 196-198° (Carter, Bhattacharyya); also reported as crystals from isopropanol, mp 200° (dec) (Marzi). Very hygroscopic solid. [a]D25 -31.3° (c = 10 in water); [a]54625 -37.0° (c = 10 in water). Sol in water, hot alcohol. Practically insol in acetone, ether, benzene.
Melting point: mp 200° (dec) (Marzi)
Optical Rotation: [a]D25 -31.3° (c = 10 in water); [a]54625 -37.0° (c = 10 in water)
Derivative Type: Hydrochloride
CAS Registry Number: 6645-46-1
Synonyms: Levocarnitine chloride
Trademarks: Flatistine (Sanofi-Aventis)
Molecular Formula: C7H15NO3.HCl
Molecular Weight: 197.66
Percent Composition: C 42.54%, H 8.16%, N 7.09%, O 24.28%, Cl 17.94%
Properties: Crystals, dec 142°.
Derivative Type: DL-Form
CAS Registry Number: 406-76-8
Synonyms: g-Amino-b-hydroxybutyric acid trimethylbetaine; g-trimethyl-b-hydroxybutyrobetaine
Literature References: Synthesis: E. Strack
et al., Ber. 86, 525 (1953); H. E. Carter, P. K. Bhattacharyya,
J. Am. Chem. Soc. 75, 2503 (1953).
Properties: Hygroscopic crystalline solid, dec 195-197°. Sol in water, ethanol.
Derivative Type: DL-Form hydrochloride
CAS Registry Number: 461-05-2
Properties: Needles from ethanol, mp 196° (dec). Very sol in water; sol in hot ethanol; slightly sol in cold ethanol. Practically insol in acetone, ether.
Melting point: mp 196° (dec)
Derivative Type: D-Form
CAS Registry Number: 541-14-0
Literature References: Prepn: S. Friedman
et al., Arch. Biochem. Biophys. 66, 10 (1957).
Properties: Crystals, dec 210-212°. [a]D +30.9°. Very sol in water and alcohol. Practically insol in acetone and ether.
Optical Rotation: [a]D +30.9°
Derivative Type: D-Form hydrochloride
CAS Registry Number: 10017-44-4
Properties: Crystals, dec 142°.
Therap-Cat: Vitamin (enzyme cofactor).
Keywords: Enzyme Cofactor; Vitamin/Vitamin Source.