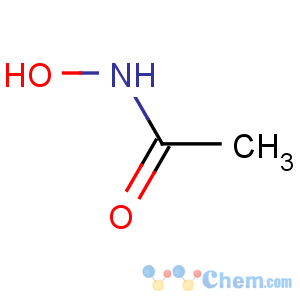
Title: Acetohydroxamic Acid
CAS Registry Number: 546-88-3
CAS Name: N-Hydroxyacetamide
Synonyms: N-acetylhydroxylamine; acetic acid oxime; AHA
Trademarks: Lithostat (Mission Pharmacal)
Molecular Formula: C2H5NO2
Molecular Weight: 75.07
Percent Composition: C 32.00%, H 6.71%, N 18.66%, O 42.63%
Line Formula: CH3CONHOH
Literature References: Urease inhibitor. Prepn: A. Miolati,
Ber. 25, 699 (1892); W. M. Wise, W. W. Brandt,
J. Am. Chem. Soc. 77, 1058 (1955); H. A. Staab
et al., Ber. 95, 1275 (1962); G. Sosnovsky, J. A. Krogh,
Synthesis 1980, 654. Inhibition of urease activity: K. Kobashi
et al., Biochim. Biophys. Acta 65, 380 (1962); W. N. Fishbein
et al., Nature 208, 46 (1965); W. N. Fishbein, P. P. Carbone,
J. Biol. Chem. 240, 2407 (1965); D. P. Griffith
et al., Invest. Urol. 11, 234 (1973). Metabolism: E. Wolpert
et al., Proc. Soc. Exp. Biol. Med. 136, 592 (1971); W. N. Fishbein
et al., J. Pharmacol. Exp. Ther. 186, 173 (1973). Pharmacokinetics: S. Feldman
et al., Invest. Urol. 15, 498 (1978). Clinical studies in treatment of kidney stones: D. B. Griffith
et al., J. Urol. 119, 9 (1978); A. Martelli
et al., Urology 17, 320 (1981).
Properties: mp 89-92°. pKa 8.70. pH (aq soln): 9.39.
Melting point: mp 89-92°
pKa: pKa 8.70
Therap-Cat: Antiurolithic. Antibacterial adjunct (urinary tract infection).
Keywords: Antiurolithic.