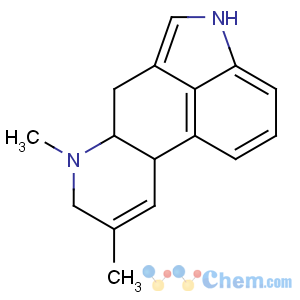
Title: Agroclavine
CAS Registry Number: 548-42-5
CAS Name: 8,9-Didehydro-6,8-dimethylergoline
Molecular Formula: C16H18N2
Molecular Weight: 238.33
Percent Composition: C 80.63%, H 7.61%, N 11.75%
Literature References: A non-peptide ergot alkaloid obtained from cultures of fungi parasitic on
Elymus mollis Trin.: Abe
et al., JP 49 178336 (1949 to Takeda),
C.A. 45, 6352c (1951);
Annu. Rep. Takeda Res. Lab. 10, 145, 167, 171 (1951);
JP 54 7498 (1954),
C.A. 50, 6000b (1956);
US 2835675 (1958). Found in fungi parasitic on
Pennisetum typhoideum Rich.: Stoll
et al., Helv. Chim. Acta 37, 1815 (1954). Structure and stereochemistry: Schreier,
ibid. 41, 1984 (1958). Biosynthesis: Floss
et al., J. Am. Chem. Soc. 90, 6500 (1968). Synthesis: Plieninger
et al., Ann. 743, 95 (1971). Metabolism: Ramstad,
Lloydia 31, 327 (1968).
Properties: Rods from ether, dec 198-203°; needles from acetone, dec 205-206°. [a]D20 -155° (c = 0.9 in chloroform); [a]D20 -182° (c = 0.5 in pyridine). uv max: 225, 284, 293 nm (e 4.47, 3.88, 3.81). Freely sol in alc, chloroform, pyridine; sol in benzene, ether; very slightly sol in water.
Optical Rotation: [a]D20 -155° (c = 0.9 in chloroform); [a]D20 -182° (c = 0.5 in pyridine)
Absorption maximum: uv max: 225, 284, 293 nm (e 4.47, 3.88, 3.81)