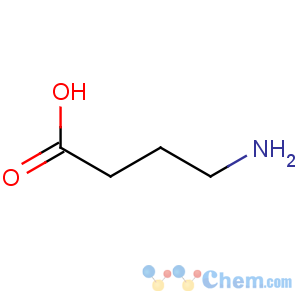
Title: g-Aminobutyric Acid
CAS Registry Number: 56-12-2
CAS Name: 4-Aminobutanoic acid
Synonyms: g-amino-
n-butyric acid; piperidic acid; GABA
Trademarks: Gammalon (Daiichi)
Molecular Formula: C4H9NO2
Molecular Weight: 103.12
Percent Composition: C 46.59%, H 8.80%, N 13.58%, O 31.03%
Line Formula: H2NCH2CH2CH2COOH
Literature References: Nonprotein amino acid that functions as a neurotransmitter. Prepn from succinimide: Tafel, Stern,
Ber. 33, 2224 (1900); from piperylurethan and fuming nitric acid: Schotten,
Ber. 16, 643 (1883); Abderhalden,
Chem. Zentralbl. 1926, II, 779; from
N-(b-bromoethyl)phthalimide and sodiomalonic ester: Aschan,
Ber. 24, 2450 (1891); from g-chlorobutyronitrile and potassium phthalimide: Gabriel,
Ber. 22, 3335 (1889);
23, 1771 (1890); DeWitt,
Org. Synth. coll. vol. II, 25 (1943). Review of biochemical pharmacology: R. Tapia in
Handbook of Psychopharmacology, L. L. Iversen
et al., Eds. (Plenum, New York, 1975) pp 1-58; L. L. Iversen in
Psychopharmacology: A Generation of Progress, M. A. Lipton
et al., Eds. (Raven, New York, 1978) pp 25-38; C. C. Mao, E. Costa,
ibid. pp 307-318.
Properties: Leaflets from methanol + ether, needles from water + alcohol, mp 202° (dec on rapid heating). Ka 3.7 ′ 10-11; Kb 1.7 ′ 10-10 at 25°. Freely sol in water. Insol or poorly sol in other solvents. On melting it dec forming pyrrolidone and water.
Melting point: mp 202° (dec on rapid heating)
Derivative Type: Hydrochloride
Molecular Formula: C4H9NO2.HCl
Molecular Weight: 139.58
Percent Composition: C 34.42%, H 7.22%, N 10.03%, O 22.93%, Cl 25.40%
Properties: Crystals, mp 135-136°.
Melting point: mp 135-136°
Derivative Type: Ethyl ester
Molecular Formula: C6H13NO2
Molecular Weight: 131.17
Percent Composition: C 54.94%, H 9.99%, N 10.68%, O 24.39%
Properties: Liquid, bp12 76°.
Boiling point: bp12 76°
Therap-Cat: Antihypertensive.
Keywords: Antihypertensive.