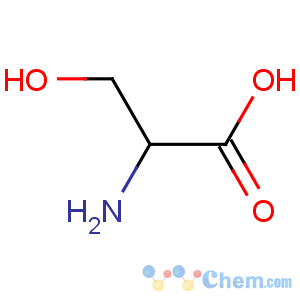
Title: Serine
CAS Registry Number: 56-45-1
CAS Name: L-Serine
Synonyms: Ser; S; 2-amino-3-hydroxypropionic acid; b-hydroxyalanine; (
S)-2-amino-3-hydroxypropanoic acid; a-amino-b-hydroxypropionic acid
Molecular Formula: C3H7NO3
Molecular Weight: 105.09
Percent Composition: C 34.29%, H 6.71%, N 13.33%, O 45.67%
Literature References: A non-essential amino acid for human development. A major intracellular source of one carbon units for
de novo purine synthesis. Found in the active site of serine proteases such as trypsin. Isoln from the silk protein, sericine: E. Cramer,
Prakt. Chem. 96, 76 (1865). Structure and synthesis as DL-form: E. Fischer, H. Leuchs,
Ber. 35, 3787 (1902). Early chemistry and biochemistry:
Amino Acids and Proteins, D. M. Greenberg, Ed. (Charles C. Thomas, Springfield, IL, 1951) 950 pp.,
passim; J. P. Greenstein, M. Winitz,
Chemistry of the Amino Acids vols 1-3 (John Wiley and Sons, Inc., New York, 1961) pp. 2202-2237,
passim. Enzymatic determn in biological samples: R. D. Hurst
et al., Anal. Biochem. 117, 339 (1981). Synthetic reviews: K. Toi in
Synthetic Production and Utilization of Amino Acids, T. Kaneko
et al., Eds. (Halsted, New York, 1974) pp 187-195; of DL-forms: L. Bassignani
et al., Ber. 112, 148-160 (1979). Review of role in enzyme active sites: B. S. Hartley,
Ann. N.Y. Acad. Sci. 227, 438-445 (1974); J. Lamotte-Brasseur
et al., Biotechnol. Genet. Eng. Rev. 12, 189-230 (1994). Review of metabolism: K. Snell, D. A. Fell,
Adv. Enzyme Regul. 30, 13-32 (1990).
Properties: Hexagonal plates or prisms. Sweetish taste, insipid aftertaste. Dec 228°. Sublimes at 150° in high vac (10-4 mm Hg). [a]D20 -6.83° (c = 10.41); [a]D25 +14.95° (c = 9.34 in 1
N HCl). Absorption spectrum:
Z. Physiol. Chem. 176, 257 (1928). Sol in water. Insol in common neutral solvents.
Optical Rotation: [a]D20 -6.83° (c = 10.41); [a]D25 +14.95° (c = 9.34 in 1
N HCl)
Derivative Type: DL-Form
CAS Registry Number: 302-84-1
Properties: Monoclinic prismatic leaflets from water. d 1.537. Dec 246° (closed capillary, bath preheated to 225°). Sublimes at 150° in high vac (10-4 mm Hg). pK1 2.21; pK2 9.15. Soly in water (g/l) at 0° = 22.04; at 25° = 50.23; at 50° = 103; at 75° = 192; at 100° = 322. Insol in common neutral solvents.
pKa: pK1 2.21; pK2 9.15
Density: d 1.537
Derivative Type: D-Form
CAS Registry Number: 312-84-5
Literature References: Presence in rat brain: A. Hashimoto
et al., FEBS Lett. 296, 33 (1992). As synaptic modulator: M. J Schell
et al., Proc. Natl. Acad. Sci. USA 92, 3948 (1995).