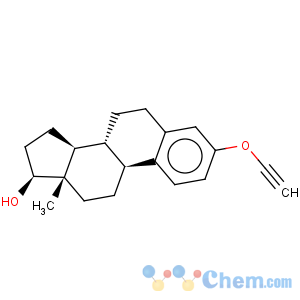
Title: Ethinyl Estradiol
CAS Registry Number: 57-63-6
CAS Name: (17a)-19-Norpregna-1,3,5(10)-trien-20-yne-3,17-diol
Synonyms: 17a-ethynyl-1,3,5(10)-estratriene-3,17b-diol; 17-ethinylestradiol; ethynylestradiol
Trademarks: Estinyl (Schering-Plough); Feminone (Pharmacia & Upjohn); Lynoral (Organon); Orestralyn (McNeil); Primogyn C (Schering AG); Progynon C (Schering AG)
Molecular Formula: C20H24O2
Molecular Weight: 296.40
Percent Composition: C 81.04%, H 8.16%, O 10.80%
Literature References: Synthetic steroid with high oral estrogenic potency: Inhoffen, Hohlweg,
Naturwissenschaften 26, 96 (1938). Prepn from estrone: Inhoffen
et al., Ber. 71, 1024 (1938).
See also DE 702063;
GB 516444;
US 2243887;
US 2251939;
US 2265976;
US 2267257. Properties: Petit, Muller,
Bull. Soc. Chim. Fr. 1951, 121; L. Ehmann, A. Wettstein,
Pharm. Acta Helv. 25, 297 (1950). NMR: Hampel, Kraemer,
Ber. 98, 3255 (1965). Toxicity: E. I. Goldenthal,
Toxicol. Appl. Pharmacol. 18, 185 (1971). Randomized double-blind clinical studies: S. Koetsawang
et al., Contraception 25, 231 (1982); A. Sheth
et al., ibid. 243. Clinical evaluation in gonadal dysgenesis: L. Cuttler
et al., J. Clin. Endocrinol. Metab. 60, 1087 (1985). General review: K. W. Thompson,
J. Clin. Pharmacol. 8, 1088-1098 (1948). Review of metabolism and pharmacokinetics: K. Fotherby,
Methods Find. Exp. Clin. Pharmacol. 4, 133-141 (1982); of carcinogenicity studies:
IARC Monographs 21, 233-255 (1979);
ibid. Suppl. 4, 186-188 (1982).
Derivative Type: Hemihydrate
Properties: Fine needles from methanol + water, mp 141-146°, [a]D25 0 ± 1° (dioxane). Dehydrates after melting and further heating, mp 182-184°. [a]D24 +3.5 ± 0.5° (c = 2 in dioxane); -29.5 ± 1° (c = 2 in pyridine). uv max (ethanol): 281 nm (e 2040 ± 60). Practically insol in water. Soly: 1 part in 6 of ethanol, 1 in 4 of ether, 1 in 5 of acetone, 1 in 4 of dioxane, and 1 in 20 of chloroform. Sol in vegetable oils, and in solns of fixed alkali hydroxides. LD50 in rats, mice (mg/kg): 2952, 1737 orally (Goldenthal).
Melting point: mp 141-146°; mp 182-184°
Optical Rotation: [a]D25 0 ± 1° (dioxane); [a]D24 +3.5 ± 0.5° (c = 2 in dioxane); -29.5 ± 1° (c = 2 in pyridine)
Absorption maximum: uv max (ethanol): 281 nm (e 2040 ± 60)
Toxicity data: LD50 in rats, mice (mg/kg): 2952, 1737 orally (Goldenthal)
Derivative Type: 3-Acetate
Molecular Formula: C22H26O3
Molecular Weight: 338.44
Percent Composition: C 78.07%, H 7.74%, O 14.18%
Properties: Crystals, mp 152-153°. [a]D20 +3° (chloroform).
Melting point: mp 152-153°
Optical Rotation: [a]D20 +3° (chloroform)
Derivative Type: 3-Benzoate
Molecular Formula: C27H28O3
Molecular Weight: 400.51
Percent Composition: C 80.97%, H 7.05%, O 11.98%
Properties: Needles from methanol, mp 200-202°.
Melting point: mp 200-202°
NOTE: Also used in combination with chlormadinone acetate, desogestrel, ethynodiol, gestodene, lynestrenol, norethindrone or norgestrel,
q.q.v. Has been used in combination with dimethisterone, medroxyprogesterone, or megestrol acetate,
q.q.v.
CAUTION: These substances are listed as known human carcinogens:
Report on Carcinogens, Eleventh Edition (PB2005-104914, 2004) p III-115.
Therap-Cat: Estrogen. In combination with progestogen as oral contraceptive.
Therap-Cat-Vet: Estrogen.
Keywords: Contraceptive (Oral); Estrogen; Steroidal.