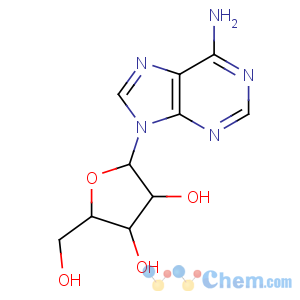
References of (2R,3R,4S,5R)-2-(6-aminopurin-9-yl)-5-(hydroxymethyl)oxolane-3,4-diol
Title: Adenosine
CAS Registry Number: 58-61-7
CAS Name: 9-b-D-Ribofuranosyl-9
H-purin-6-amine
Synonyms: 6-amino-9-b-D-ribofuranosyl-9
H-purine; 9-b-D-ribofuranosidoadenine; adenine riboside
Trademarks: Adenocard (Fujisawa); Adenocor (Sanofi Winthrop); Adenoscan (Fujisawa)
Molecular Formula: C10H13N5O4
Molecular Weight: 267.24
Percent Composition: C 44.94%, H 4.90%, N 26.21%, O 23.95%
Literature References: Nucleoside; widely distributed in nature. From yeast nucleic acid: Levene and Bass,
Nucleic Acids (New York, 1931) p 163. Structure: Levene, Tipson,
J. Biol. Chem. 94, 809 (1932); Bredereck,
Ber. 66, 198 (1933);
Z. Physiol. Chem. 223, 61 (1934); Gulland, Holiday,
J. Chem. Soc. 1936, 765.
Cf. Szent-Gy?rgyi,
J. Physiol. 68, 213 (1930); Lythgoe
et al., J. Chem. Soc. 1947, 355;
1948, 965. Synthesis: Davoll
et al., ibid. 1948, 967; H. Vorbrueggen, K. Krolikiewicz,
Angew. Chem. Int. Ed. 14, 421 (1975). Crystal structure: T. F. Lai, R. E. Marsh,
Acta Crystallogr. B28, 1982 (1972). Conformational properties: D. B. Davies, A. Rabczenko,
J. Chem. Soc. Perkin Trans. 2 1975, 1703. Symposium on cardiac electrophysiology, pharmacology and clinical efficacy in supraventricular tachycardia:
Prog. Clin. Biol. Res. 230, 1-395 (1987).
Reviews: see Adenine.
Properties: Crystals from water, mp 234-235°. [a]D11 -61.7° (c = 0.706 in water); [a]9D -58.2° (c = 0.658 in water). uv max: 260 nm (e 15100). Practically insol in alcohol.
Melting point: mp 234-235°
Optical Rotation: [a]D11 -61.7° (c = 0.706 in water); [a]9D -58.2° (c = 0.658 in water)
Absorption maximum: uv max: 260 nm (e 15100)
Therap-Cat: Antiarrhythmic.
Keywords: Antiarrhythmic.