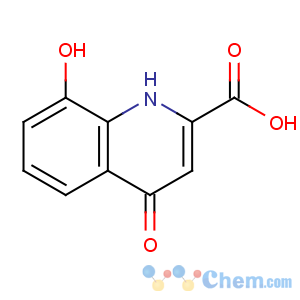
Title: Xanthurenic Acid
CAS Registry Number: 59-00-7
CAS Name: 4,8-Dihydroxy-2-quinolinecarboxylic acid
Synonyms: 4,8-dihydroxyquinaldic acid
Molecular Formula: C10H7NO4
Molecular Weight: 205.17
Percent Composition: C 58.54%, H 3.44%, N 6.83%, O 31.19%
Literature References: Excreted by pyridoxine-deficient animals after the ingestion of tryptophan. Isoln from the urine of albino rats fed almost exclusively on fibrin: Musajo,
Gazz. Chim. Ital. 67, 165 (1937). Synthesis from ethyl oxalacetate and
o-anisidine: Musajo, Minchilli,
Ber. 74, 1842 (1941); Mebane, Oroshnik,
J. Am. Chem. Soc. 73, 3520 (1951); Furst, Olsen,
J. Org. Chem. 16, 412 (1951).
Properties: Sulfur-yellow crystals, mp 286°. uv max (water): 243, 342 nm (e 30000, 6500). Insol in water. Sol in aq alkali hydroxides and carbonates (yellow solns) and in hot dil HCl. It gives a red color with Millon reagent, intense ruby-red with alkali diazobenzenesulfonates, and intense green with FeSO4. When the acid is dissolved in very dil NaHCO3, the green color with FeSO4 is still visible at a diln of 1:200,000.
Melting point: mp 286°
Absorption maximum: uv max (water): 243, 342 nm (e 30000, 6500)
Derivative Type: Methyl ester
Molecular Formula: C11H9NO4
Molecular Weight: 219.19
Percent Composition: C 60.28%, H 4.14%, N 6.39%, O 29.20%
Properties: Yellow crystals, dec 262°. Sol in NH4OH, alkali hydroxides and carbonates.