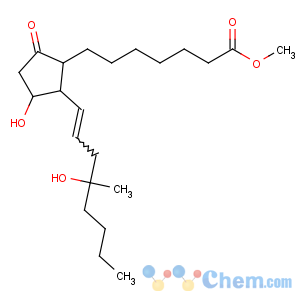
References of Prost-13-en-1-oic acid,11,16-dihydroxy-16-methyl-9-oxo-, methyl ester, (11a,13E)-(?à)-
Title: Misoprostol
CAS Registry Number: 59122-46-2
CAS Name: (11a,13
E)-11,16-Dihydroxy-16-methyl-9-oxoprost-13-en-1-oic acid methyl ester
Synonyms: (±)-methyl-(1
R,2
R,3
R)-3-hydroxy-2-[
(E)-(4
RS)-4-hydroxy-4-methyl-1-octenyl]-5-oxocyclopentaneheptanoate; (±)-15-deoxy-(16
RS)-16-hydroxy-16-methyl-PGE1 methyl ester
Manufacturers' Codes: SC-29333
Trademarks: Cytotec (Pfizer)
Molecular Formula: C22H38O5
Molecular Weight: 382.53
Percent Composition: C 69.08%, H 10.01%, O 20.91%
Literature References: Cytoprotective prostaglandin PGE1 analog; also exhibits uterotonic and cervical-ripening actions. Double racemate comprised of the (+)- and (-)-enantiomers of the 16
R- and 16
S-forms. The pharmacologically active form is the (11
R,16
S)-enantiomer. Prepn: P. W. Collins, R. Pappo,
BE 827127;
eidem, US 3965143 (1975, 1976 both to Searle); P. W. Collins
et al., Tetrahedron Lett. 48, 4217 (1975). Prepn, activity, NMR data: P. Collins
et al., J. Med. Chem. 20, 1152 (1977). HPLC resolution of enantiomers: D. A. Roston, R. Wijayaratne,
Anal. Chem. 60, 948 (1988). Mechanism of gastric secretory inhibition: D. G. Colton
et al., Arch. Int. Pharmacodyn. Ther. 236, 86 (1978). Symposium on pharmacology and clinical efficacy:
Dig. Dis. Sci. 30, Suppl. 11, 114S-205S (1985). Toxicity data: F. N. Kotsonis
et al., ibid. 142S. Clinical trial in prevention of NSAID-induced ulcer: D. Y. Graham
et al., Ann. Intern. Med. 119, 257 (1993); to induce labor: H. Fletcher
et al., Obstet. Gynecol. 83, 244 (1994). Review of clinical experience in pregnancy: A. B. Goldberg
et al., N. Engl. J. Med. 344, 38-47 (2001).
Properties: Light yellow oil. Sol in water. LD50 in rats, mice (mg/kg): 40-62, 70-160 i.p.; 81-100, 27-138 orally (Kotsonis).
Toxicity data: LD50 in rats, mice (mg/kg): 40-62, 70-160 i.p.; 81-100, 27-138 orally (Kotsonis)
Therap-Cat: Antiulcerative.
Keywords: Antiulcerative; Prostaglandin/Prostaglandin Analog.