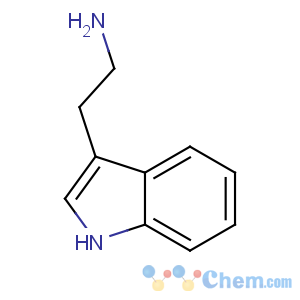
Title: Tryptamine
CAS Registry Number: 61-54-1
CAS Name: 1
H-Indole-3-ethanamine
Synonyms: 3-(2-aminoethyl)indole; 2-(3-indolyl)ethylamine
Molecular Formula: C10H12N2
Molecular Weight: 160.22
Percent Composition: C 74.96%, H 7.55%, N 17.48%
Literature References: Occurs in plants. Synthesis starting with nitroethylene and indole: Noland, Hartman,
J. Am. Chem. Soc. 76, 3227 (1954). Alternate routes: Thesing, Schulde,
Ber. 85, 324 (1952); Jackson, Smith,
J. Chem. Soc. 1965, 3498; Tacconi,
Farmaco Ed. Sci. 20, 902 (1965); S. Takano
et al., Heterocycles 6, 1167 (1977); I. Fleming, M. Woolias,
J. Chem. Soc. Perkin Trans. 1 1979, 829. X-ray structure determn: Wakahara
et al., Tetrahedron Lett. 1970, 4999.
Review of tryptamine syntheses: J. E. Saxton in R. H. F. Manske,
The Alkaloids vol. VIII (1965) pp 8-10.
Properties: Needles from petr ether, mp 118°. uv max (ethanol): 222, 282, 290 nm (log e 4.56, 3.78, 3.71). Sol in ethanol, acetone. Practically insol in water, ether, benzene, chloroform.
Melting point: mp 118°
Absorption maximum: uv max (ethanol): 222, 282, 290 nm (log e 4.56, 3.78, 3.71)
Derivative Type: Hydrochloride
Molecular Formula: C10H12N2.HCl
Molecular Weight: 196.68
Percent Composition: C 61.07%, H 6.66%, N 14.24%, Cl 18.03%
Properties: Needles from ethanol + ethyl acetate, mp 248°. uv max (95% ethanol): 221, 275, 281, 290 nm (log e 4.52, 3.73, 3.75, 3.69).
Melting point: mp 248°
Absorption maximum: uv max (95% ethanol): 221, 275, 281, 290 nm (log e 4.52, 3.73, 3.75, 3.69)