Title: Cucurbitacins
Literature References: A group of tetracyclic triterpenes, commonly referred to as "bitter principles of cucurbits", which have antineoplastic and anti-gibberellin activity. They are isolated from various spp of cucurbitaceous plants known since antiquity for their beneficial and toxic properties. The plants have been used as vermifuges, emetics, narcotics, and antimalarials and have been implicated in sporadic livestock poisoning in S. Africa. Seventeen cucurbitacins have been isolated, most from plants of the
Cucurbitaceae family, but also from
Begoniaceae, Cruciferae, Datisceae, Euphorbiaceae, and
Scrophulariaceae. Cucurbitacins B and E are the most commonly identified. Isoln of cucurbitacins A, B, C, D, F: P. R. Enslin,
J. Sci. Food Agric. 5, 410 (1954); of G, H, J, K, L:
idem, ibid. 8, 673 (1957); of E and I: D. Lavie, S. J. Szinai,
J. Am. Chem. Soc. 80, 707 (1958); of O, P, Q and antitumor activity: S. Kupchan
et al., J. Org. Chem. 35, 2891 (1970). Structures of A, B, C, D, E, I: W. T. DeKock
et al., J. Chem. Soc. 1963, 3828; of B, D, F: D. Lavie
et al., Chem. Ind. (London) 1959, 951; of G, H: C. W. Holzapfel, P. R. Enslin,
J. S. Afr. Chem. Inst. 17, 142 (1964),
C.A. 62, 10467c (1965); of J, K, L: P. R. Enslin, K. B. Norton,
J. Chem. Soc. 1964, 529. Stereochemistry of B, D, E, F, I: D. Lavie
et al., J. Org. Chem. 28, 1790 (1963). 13C-NMR study of cucurbitacins: V. V. Velde, D. Lavie,
Tetrahedron 39, 317 (1983). Use as plant growth regulators: J. Guha, S. P. Sen,
Nature New Biol. 244, 273 (1973). Toxicity studies: J. LeMen
et al., Chim. Ther. 4, 459 (1969); D. Albert
et al., Chim. Ther. 5, 205 (1970).
Reviews: D. Lavie, E. Glotter,
Fortschr. Chem. Org. Naturst. 29, 308-357 (1971); J. Guha, S. P. Sen,
Plant Biochem. J. 2, 12-28 (1975); A. Shrotria,
Botanica 26, 28-31 (1976).
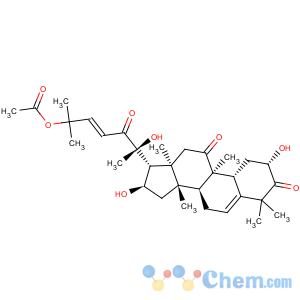
Derivative Type: Cucurbitacin B
CAS Registry Number: 6199-67-3
CAS Name: (2b,9b,10a,16a,23
E)-25-(Acetyloxy)-2,16,20-trihydroxy-9-methyl-19-norlanosta-5,23-diene-3,11,22-trione
Synonyms: 1,2-dihydro-a-elaterin
Molecular Formula: C32H46O8
Molecular Weight: 558.70
Percent Composition: C 68.79%, H 8.30%, O 22.91%
Properties: Crystals from abs ethanol, mp 184-186°. [a]D25 +88° (c = 1.55 in ethanol). LD10 orally in mice: 5 mg/kg (LeMen).
Melting point: mp 184-186°
Optical Rotation: [a]D25 +88° (c = 1.55 in ethanol)
Toxicity data: LD10 orally in mice: 5 mg/kg (LeMen)
Derivative Type: Cucurbitacin E
CAS Registry Number: 18444-66-1
CAS Name: (9b,10a,16a,23
E)-25-(Acetyloxy)-2,16,20-trihydroxy-9-methyl-19-norlanosta-1,5,23-triene-3,11,22-trione
Synonyms: a-elaterin
Molecular Formula: C32H44O8
Molecular Weight: 556.69
Percent Composition: C 69.04%, H 7.97%, O 22.99%
Properties: White hexagonal plates from methanol, mp 232-233° (dec). [a]D -59° (c = 0.7 in chloroform). uv max (chloroform): 234, 267 nm (e 11700, 8350). LD50 orally in mice: 340 mg/kg (Albert).
Melting point: mp 232-233° (dec)
Optical Rotation: [a]D -59° (c = 0.7 in chloroform)
Absorption maximum: uv max (chloroform): 234, 267 nm (e 11700, 8350)
Toxicity data: LD50 orally in mice: 340 mg/kg (Albert)