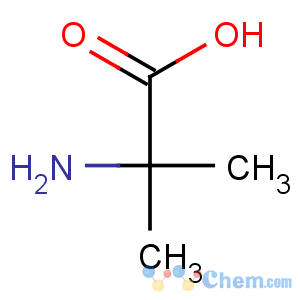
Title: a-Aminoisobutyric Acid
CAS Registry Number: 62-57-7
CAS Name: 2-Methylalanine
Synonyms: 2-aminoisobutyric acid; 2-amino-2-methylpropanoic acid
Molecular Formula: C4H9NO2
Molecular Weight: 103.12
Percent Composition: C 46.59%, H 8.80%, N 13.58%, O 31.03%
Line Formula: (CH3)2C(NH2)COOH
Literature References: Prepd by the treatment of acetone with hydrocyanic acid and then with alcoholic ammonia (Strecker synthesis): Tiemann, Friedl?nder,
Ber. 14, 1970 (1881),
see also p 1965; Marckwald
et al., Ber. 24, 3283 (1891); Bailey, Randolph,
Ber. 41, 2507 (1908); Clarke, Bean,
Org. Synth. coll. vol. II, 29 (1943); or directly with ammonium cyanide: Gulewitsch,
Ber. 33, 1900 (1900) or with a mixture of KCN and NH4Cl: Zelinsky, Stadnikow,
Ber. 39, 1726 (1906);
cf. Hellsing,
Ber. 37, 1921 (1904); and subsequent hydrolysis of the nitrile formed. By heating dimethylhydantoin (obtained from acetone, hydrocyanic and cyanic acids) with concd HCl: Urech,
Ann. 164, 268 (1872),
cf. Heilpern,
Monatsh. Chem. 17, 241 (1896).
Properties: Monoclinic prisms, tables, mp 335° (sealed capillary). Begins to sublime at 280°. Sweetish taste. Absorption spectrum: Ley, Arends,
Ber. 61, 219 (1928); Abderhalden, Rossner,
Z. Physiol. Chem. 176, 253 (1928). Freely sol in water. Difficultly sol in alcohol; insol in ether.
Melting point: mp 335° (sealed capillary)
Derivative Type: Hydrochloride
Molecular Formula: C4H9NO2.HCl
Molecular Weight: 139.58
Percent Composition: C 34.42%, H 7.22%, N 10.03%, O 22.93%, Cl 25.40%
Properties: Platelets from water, dec 236-237°. Readily sol in water, methanol, alcohol.