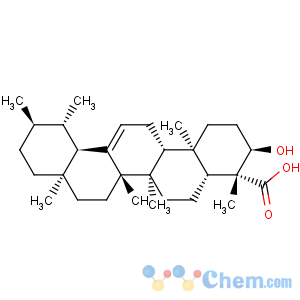
Title: b-Boswellic Acid
CAS Registry Number: 631-69-6
CAS Name: (3a,4b)-3-Hydroxyurs-12-en-23-oic acid
Molecular Formula: C30H48O3
Molecular Weight: 456.70
Percent Composition: C 78.90%, H 10.59%, O 10.51%
Literature References: Occurs as the acetate in frankincense
(olibanum) from
Boswellia carterii, Burseraceae. The b-form is predominant and is accompanied by small amounts of a- and g-boswellic acid. Isoln from olibanum tears: Winterstein, Stein,
Z. Physiol. Chem. 208, 9 (1932). Early structural studies: Simpson, Williams,
ibid. 1938, 686, 1712; Ruzicka, Wirz,
Helv. Chim. Acta 22, 948 (1939);
23, 132 (1940); Ruzicka
et al., ibid. 27, 1859 (1944). Revised structure and stereochemistry: Beton
et al., J. Chem. Soc. 1956, 2904; Allan,
Chimia 17, 382 (1963);
idem, Phytochemistry 7, 963 (1968).
Review: J. Simonsen, W. C. J. Ross,
The Terpenes vol. 5 (University Press, Cambridge, 1957) pp 68-74.
Properties: Long prisms from methanol, mp 228-232° with preliminary sintering. [a]D +107° (c = 0.75 in CHCl3). 100 ml of boiling methanol will dissolve 8 grams of b-boswellic acid. Sol in chloroform, ether, acetone, alc.
Melting point: mp 228-232° with preliminary sintering
Optical Rotation: [a]D +107° (c = 0.75 in CHCl3)
Derivative Type: Acetate
Molecular Formula: C32H50O4
Molecular Weight: 498.74
Percent Composition: C 77.06%, H 10.10%, O 12.83%
Properties: Prisms, mp 275-278°, [a]D +63° (c = 1.88 in CHCl3).
Melting point: mp 275-278°
Optical Rotation: [a]D +63° (c = 1.88 in CHCl3)
Derivative Type: Methyl ester
Molecular Formula: C31H50O3
Molecular Weight: 470.73
Percent Composition: C 79.10%, H 10.71%, O 10.20%
Properties: mp 195-196°, [a]D +111° (c = 1.6 in CHCl3).
Melting point: mp 195-196°
Optical Rotation: [a]D +111° (c = 1.6 in CHCl3)