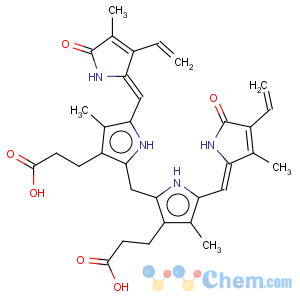
Title: Bilirubin
CAS Registry Number: 635-65-4
CAS Name: 2,17-Diethenyl-1,10,19,22,23,24-hexahydro-3,7,13,18-tetramethyl-1,19-dioxo-21
H-biline-8,12-dipropanoic acid
Synonyms: 1,10,19,22,23,24-hexahydro-2,7,13,17-tetramethyl-1,19-dioxo-3,18-divinylbiline-8,12-dipropionic acid; 1,3,6,7-tetramethyl-4,5-dicarboxyethyl-2,8-divinyl-(
b-13)-dihydrobilenone; bilirubin IXa
Molecular Formula: C33H36N4O6
Molecular Weight: 584.66
Percent Composition: C 67.79%, H 6.21%, N 9.58%, O 16.42%
Literature References: Principal pigment of bile and constituent of many biliary calculi. Major end-product of the biological breakdown of heme,
q.v. Bilirubin is the chromophore responsible for coloration in various forms of jaundice. Also found in blood serum, where it exists in four major forms: unconjugated bilirubin, the monoglucuronide, the diglucuronide, and albumin-bound bilirubin. Most easily obtained from ox gallstones which are largely calcium bilirubinate: St?deler,
Ann. 132, 323 (1864); Küster,
Z. Physiol. Chem. 94, 136 (1915);
99, 86 (1917);
121, 80 (1922); Küster, Haas,
ibid. 141, 279 (1924); Fischer,
ibid. 3, 204 (1911); Fischer, Hess,
ibid. 194, 193 (1931). Isoln from pig bile: Gibson, Lowe,
J. Biol. Chem. 123, XLI (1938); Gray
et al., J. Chem. Soc. 1961, 2264, 2268; from ox bile: Libowitzky,
Z. Physiol. Chem. 263, 267 (1940). Industrial isoln from ox bile using chlorobenzene as extractant:
US 2166073;
US 2331574;
US 2363471;
US 2386716. Structure and synthesis: Fischer, Plieninger,
Naturwissenschaften 30, 382 (1942);
Z. Physiol. Chem. 274, 231 (1942);
cf. Fischer-Orth,
Die Chemie des Pyrrols II, 1, 621 (Leipzig, 1937); Gray
et al., Nature 181, 183 (1958);
eidem, J. Chem. Soc. 1961, 2276. Structure: Fog, Jellum,
Nature 198, 88 (1963). Configuration: Kuenzle
et al., Biochem. J. 133, 364 (1973). X-ray analysis and structure: Bonnett
et al., J. Chem. Soc. Perkin Trans. 2 1972, 902, 1335;
Nature 262, 326 (1976). NMR conformation studies: D. Kaplan, G. Navon,
J. Chem. Soc. Perkin Trans. 2 1981, 1374. Separation of bilirubin species in serum and bile by reversed-phase HPLC: J. J. Lauff
et al., J. Chromatogr. 226, 391 (1981);
eidem, Clin. Chem. 29, 800 (1983). Clinical importance of albumin-bound bilirubin: J. S. Weiss
et al., N. Engl. J. Med. 309, 148 (1983). Comprehensive reviews: Lemberg, Legge,
Hematin Compounds and Bile Pigments (New York, 1949); With,
Bile Pigments (Academic Press, New York, 1968). Reviews of toxicity: D. Bratlid,
N. Y. State J. Med. 91, 489-493 (1991); R. P. Wennberg,
ibid. 493-496.
Properties: Light orange to deep reddish-brown monoclinic rhomboid, prisms, plates from chloroform. Gradually blackens on heating and does not melt. The greenish solns show a red fluorescence in ultraviolet light. A 0.001% soln in chloroform shows selective abs from 490 to 400 nm with a max at 453 nm; e mM 60.7 ± 0.8. Practically insol in water. Sol in benzene, chloroform, chlorobenzene, carbon disulfide, acids, alkalies; slightly sol in alcohol, ether. Spreads on water. Penetrates into cholesterol, octadecylamine, and protein monolayers: Stenhagen, Rideal,
Biochem. J. 33, 1591 (1939).