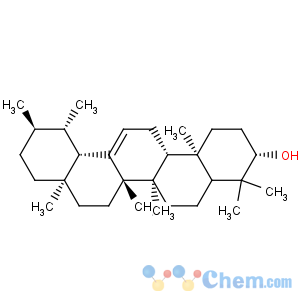
Title: a-Amyrin
CAS Registry Number: 638-95-9
CAS Name: (3b)-Urs-12-en-3-ol
Synonyms: a-amyrenol; viminalol
Molecular Formula: C30H50O
Molecular Weight: 426.72
Percent Composition: C 84.44%, H 11.81%, O 3.75%
Literature References: Occurs mostly as acetate in latex of rubber trees, in latex from
Ficus variegata Blume,
Moraceae, also in
Balanophora elongata Blume,
Balanophoraceae, and in
Erythroxylum coca Lam. var.
novogranatense Morris, and var.
spruceanum Burck,
Erythroxylaceae. Isoln from
Manila elemi: Vesterberg, Westerlind,
Ann. 428, 247 (1922). Structural studies: Spring, Vickerstaff,
J. Chem. Soc. 1937, 249; Beynon
et al., ibid. 1938, 1233; Meisels
et al., Helv. Chim. Acta 32, 1075 (1949),
38, 1298 (1955); Melera
et al., ibid. 39, 441 (1956). Identity with viminalol: Soldin, Marais,
J. Pharm. Soc. 55, 452 (1966). Formation from ursolic acid: Goodson,
J. Chem. Soc. 1938, 999; from boswellic acid: Ruzicka, Wirz,
Helv. Chim. Acta 22, 948 (1939). Partial synthesis from glycyrrhetic acid and stereochemistry: Corey, Cantrall,
J. Am. Chem. Soc. 81, 1745 (1959).
Review: J. Simonsen, W. C. J. Ross,
The Terpenes vol. IV (University Press, Cambridge, 1957) pp 116-148.
Properties: Needles from alcohol, mp 186°. bp0.7 243°. [a]D17 +91.6° (c = 1.3 in benzene). Sol in 22 parts 98% alc. Sol in ether, benzene, chloroform, glacial acetic acid. Slightly sol in petr ether.
Melting point: mp 186°
Boiling point: bp0.7 243°
Optical Rotation: [a]D17 +91.6° (c = 1.3 in benzene)
Derivative Type: Acetate
Molecular Formula: C32H52O2
Molecular Weight: 468.75
Percent Composition: C 81.99%, H 11.18%, O 6.83%
Properties: Leaflets from petr ether, mp 227°. [a]D20 +76.35° (c = 0.572 in CHCl3).
Melting point: mp 227°
Optical Rotation: [a]D20 +76.35° (c = 0.572 in CHCl3)
Derivative Type: Benzoate
Molecular Formula: C37H54O2
Molecular Weight: 530.82
Percent Composition: C 83.72%, H 10.25%, O 6.03%
Properties: Prisms from benzene + acetone, mp 195-196°. [a]D10 +94.6° (c = 1.9 in CHCl3).
Melting point: mp 195-196°
Optical Rotation: [a]D10 +94.6° (c = 1.9 in CHCl3)