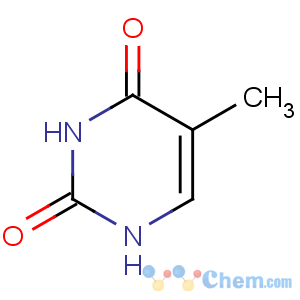
Title: Thymine
CAS Registry Number: 65-71-4
CAS Name: 5-Methyl-2,4(1
H,3
H)-pyrimidinedione
Synonyms: 5-methyluracil; 2,4-dihydroxy-5-methylpyrimidine
Molecular Formula: C5H6N2O2
Molecular Weight: 126.11
Percent Composition: C 47.62%, H 4.80%, N 22.21%, O 25.37%
Literature References: A pyrimidine derivative; constituent of nucleic acids. Originally isolated from thymus nucleic acid: Levene,
Z. Physiol. Chem. 39, 4 (1903). Prepn by heating 2-ethylmercapto-4-hydroxy-5-methylpyrimidine: Wheeler, Merriam,
Am. Chem. J. 29, 478 (1903);
43, 29 (1910). From methylcyanacetylurea by catalytic reduction: Bergmann, Johnson,
J. Am. Chem. Soc. 55, 1733 (1933). From b-methylmalic acid: Scherp,
J. Am. Chem. Soc. 68, 912 (1946). Crystal structure of monohydrate: Gerdil,
Acta Crystallogr. 14, 333 (1961).
Review: Ts'o, "Bases, Nucleosides and Nucleotides" in
Basic Principles in Nucleic Acid Chemistry vol. 1, P. O. P. Ts'o, Ed. (Academic Press, New York, 1974) pp 453-584.
Properties: Dendritic or star-shaped plates from water, sometimes short needles. Sublimes in platelets. Dec 335-337° (Kofler stage). Weak acid, pK at 25° = 9.94. uv max (pH 7.0): 205, 264.5 nm (e ′ 103 9.5, 7.9). Absorption spectra: D. Voet
et al., Biopolymers 1, 193 (1963). Sol in hot water; slightly sol in cold water (4 g/l at 25°). Somewhat sol in alc; sparingly sol in ether; readily sol in alkalies with formation of salts. Oxidation yields urea, ethanal, pyruvic acid, formic acid. Hydrazine reacts with thymine forming urea and 4-methylpyrazolone. Thymine forms a silver salt which is sol in excess ammonia. Its mercuric and lead salts are insol.
pKa: pK at 25° = 9.94
Absorption maximum: uv max (pH 7.0): 205, 264.5 nm (e ′ 103 9.5, 7.9)
Derivative Type: Thymine-2-desoxyriboside
see Thymidine
Use: In biochemical research.