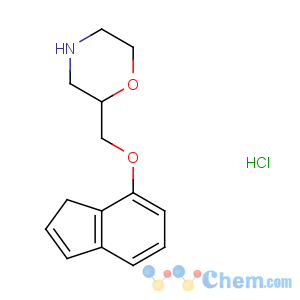
Title: Indeloxazine Hydrochloride
CAS Registry Number: 65043-22-3
CAS Name: 2-[(1
H-Inden-7-yloxy)methyl]morpholine hydrochloride
Synonyms: (±)-2-[(7-indenyloxy)methyl]morpholine hydrochloride
Manufacturers' Codes: YM-08054-1
Trademarks: Elen (Yamanouchi); Noin (Schering)
Molecular Formula: C14H18ClNO2
Molecular Weight: 267.75
Percent Composition: C 62.80%, H 6.78%, Cl 13.24%, N 5.23%, O 11.95%
Literature References: Serotonin uptake inhibitor. Prepn: M. Masuo
et al., DE 2601703;
eidem, US 4109088 (1976, 1978 both to Yamanouchi). Synthesis and resolution of isomers: T. Kojima
et al., Chem. Pharm. Bull. 33, 3766 (1985). Pharmacology and toxicity: S. Tachikawa
et al., Arch. Int. Pharmacodyn. 238, 81 (1979). Inhibition of synaptosomal uptake of serotonin and noradrenaline: M. Harada, H. Maeno,
Biochem. Pharmacol. 28, 2645 (1979). GLC determn in human plasma: A. G. Hayes, T. Chang,
J. Chromatogr. 272, 176 (1983).
Properties: Polymorphic; pale yellow needles from methanol, mp 169-170°; colorless, acicular crystals from acetone, mp 155-156°. LD50 in mice (mg/kg): 47 i.v. (Tachikawa).
Melting point: mp 169-170°; mp 155-156°
Toxicity data: LD50 in mice (mg/kg): 47 i.v. (Tachikawa)
Derivative Type: (+)-Form
Properties: Crystals from ethanol, mp 112-113°. [a]D21 +4.9° (c = 5 in methanol).
Melting point: mp 112-113°
Optical Rotation: [a]D21 +4.9° (c = 5 in methanol)
Derivative Type: (-)-Form
Properties: Crystals from isopropanol, mp 142-142.5°. [a]D20 -4.9° (c = 5 in methanol).
Melting point: mp 142-142.5°
Optical Rotation: [a]D20 -4.9° (c = 5 in methanol)
Therap-Cat: Antidepressant; nootropic.
Keywords: Antidepressant; Bicyclics; Nootropic; Serotonin Uptake Inhibitor.