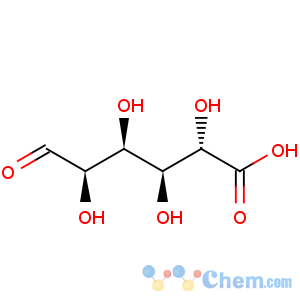
Title: D-Glucuronic Acid
CAS Registry Number: 6556-12-3
Molecular Formula: C6H10O7
Molecular Weight: 194.14
Percent Composition: C 37.12%, H 5.19%, O 57.69%
Literature References: Widely distributed in the plant and animal kingdoms. Usually occurs in "paired" form,
i.e. as a glycosidic combination with phenols, alcohols, etc. Such glucuronides form in the liver to detoxify poisonous hydroxyl-containing substances. The glucuronides present in normal urine are those of phenol, cresol, and indoxyl. After the ingestion of poisons such as morphine, chloral hydrate, camphor, or turpentine, glucuronides formed with the poison or its hydroxylated derivatives appear in the urine. Review and bibliography: Stacey,
Adv. Carbohydr. Chem. 2, 161 (1946); Jones, Smith,
ibid. 4, 243 (1949). Structure: Pryde, Williams,
Nature 128, 187 (1931); Levene, Meyer,
J. Biol. Chem. 92, 257 (1931); Levene, Kreider,
ibid. 120, 597 (1937). Review of syntheses: Mehltretter,
Adv. Carbohydr. Chem. 8, 231 (1953). Prepn by irradiation of D-glucose in dil aq soln: Phillips
et al., J. Chem. Soc. 1958, 3522; by g-irradiation of aq sucrose soln: Phillips, Moody,
ibid. 1960, 762. Electrophoretic sepn of D-glucuronic acid and its C-5 epimer,
L-iduronic acid: I. Miyamoto, S. Nagase,
Anal. Biochem. 115, 308 (1981). Monographs: N. E. Artz, E. M. Osman,
Biochemistry of Glucuronic Acid (Academic Press, New York, 1950); G. J. Dutton, Ed.,
Glucuronic Acid, Free and Combined (Academic Press, New York, 1966) 629 pp.
Derivative Type: b-Form
Properties: Needles from alcohol or ethyl acetate. mp 165°. Shows mutarotation: [a]D24 +11.7° ? +36.3° (2 hrs, c = 6). Soluble in water, alcohol. Reduces Fehling's soln.
Melting point: mp 165°
Optical Rotation: [a]D24 +11.7° ? +36.3° (2 hrs, c = 6)