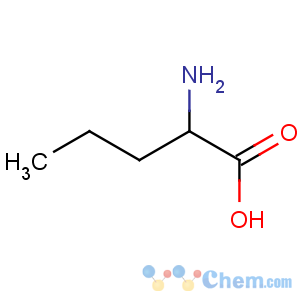
Title: Norvaline
CAS Registry Number: 6600-40-4
CAS Name: 2-Aminovaleric acid
Synonyms: a-aminovaleric acid; 2-aminopentanoic acid
Molecular Formula: C5H11NO2
Molecular Weight: 117.15
Percent Composition: C 51.26%, H 9.46%, N 11.96%, O 27.31%
Line Formula: CH3(CH2)2CH(NH2)COOH
Literature References: Prepd by treating butyraldehyde ammonia with HCN and HCl: Slimmer,
Ber. 35, 404 (1902); from 2-acetylvaleric acid ethyl ester: Hamlin, Hartung,
J. Biol. Chem. 145, 349 (1942); from acetamidomalonic acid diethyl ester: Archer, Albertson,
US 2445817 (1948 to Winthrop-Stearns); from 1-nitrobutane: Stiles, Finkbeiner,
J. Am. Chem. Soc. 85, 616 (1963);
US 3055936 (1962 to Res. Corp.). Prepn of optically active forms: Abderhalden, Kurton,
Fermentf. 4, 328;
Chem. Zentralbl. 1921, III, 296.
Derivative Type: DL-Form
Properties: Minute leaflets from alcohol or water, mp 303° (closed capillary). pK1¢ 2.36; pK2¢ 9.72. Sublimes without decompn. One gram dissolves in 10 ml water at 18°. Freely sol in hot water; practically insol in alcohol, ether, chloroform, ethyl acetate, petr ether.
Melting point: mp 303° (closed capillary)
pKa: pK1¢ 2.36; pK2¢ 9.72
Derivative Type: L(+)-Form
Properties: Crystals from dil alc. mp ~305° (closed capillary). [M]D +29.2° (5
N HCl); [M]D +41.0° (glacial acetic acid). [a]D20 +23.0° (c = 10 in 20% HCl). Freely sol in hot water; insol in alcohol, ether, chloroform, ethyl acetate, petr ether.
Melting point: mp ~305° (closed capillary)
Optical Rotation: [a]D20 +23.0° (c = 10 in 20% HCl)
Derivative Type: D(-)-Form
Properties: Minute leaflets. mp ~307°. [a]D20 -24.2° (c = 10 in 20% HCl). Freely sol in hot water; insol in alcohol, ether, chloroform, ethyl acetate, petr ether.
Melting point: mp ~307°
Optical Rotation: [a]D20 -24.2° (c = 10 in 20% HCl)