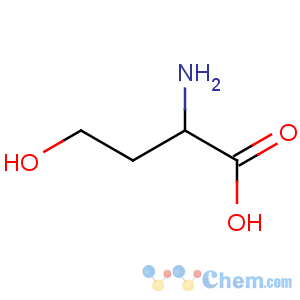
Title: Homoserine
CAS Registry Number: 672-15-1
Synonyms: 2-Amino-4-hydroxybutanoic acid; 2-amino-4-hydroxybutyric acid; a-amino-g-hydroxy-
n-butyric acid
Molecular Formula: C4H9NO3
Molecular Weight: 119.12
Percent Composition: C 40.33%, H 7.62%, N 11.76%, O 40.29%
Literature References: Principal free amino acid occurring in pea plants: A. I. Virtanen,
Acta Chem. Scand. 7, 1423 (1953); J.A. Bakhuis,
Nature 180, 713 (1957). Prepn: Fischer, Blumenthal,
Ber. 40, 106 (1907); Armstrong
J. Am. Chem. Soc. 70, 1756 (1948); Birnbaum, Greenstein,
Arch. Biochem. Biophys. 42, 212 (1953); M. Frankel, Y. Knobler,
J. Am. Chem. Soc. 80, 3147 (1958). Review of homoserine production by fermentation: T. Nara in
Microbial Prod. Amino Acids, K. Yamada, Ed. (Wiley, New York, 1972) pp 417-434.
Derivative Type: L-Homoserine
Properties: Flat prisms from 90% alc. Dec 203°. [M]D +21.8° (5
N HCl), [M]D +14.3° (glacial acetic acid). [a]D26 -8.8° (c = 5 in H2O); [a]D26 +18.3° (c = 2 in 2
N HCl). On standing for 8 hrs at 26° the [a]D of the HCl soln decreases to nearly zero as the corresponding levorotatory-g-butyrolactone is formed.
Optical Rotation: [a]D26 -8.8° (c = 5 in H2O); [a]D26 +18.3° (c = 2 in 2
N HCl)
Derivative Type: L-Homoserine g-lactone monohydrochloride
Properties: Prepd by refluxing L-homoserine with 2
N HCl for 2 hrs, crystals, [a]D26 -27.0° (c = 5).
Optical Rotation: [a]D26 -27.0° (c = 5)
Derivative Type: D-Homoserine
Properties: Crystals, dec 203°. [a]D26 +8.8° (c = 5).
Optical Rotation: [a]D26 +8.8° (c = 5)
Derivative Type: DL-Homoserine
Properties: Crystals from dil ethanol, dec 186-187°.