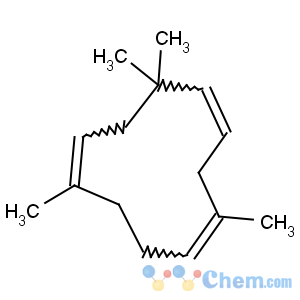
Title: Humulene
CAS Registry Number: 6753-98-6
CAS Name: (1
E,4
E,8
E)-2,6,6,9-Tetramethyl-1,4,8-cycloundecatriene
Synonyms: a-humulene; a-caryophyllene
Molecular Formula: C15H24
Molecular Weight: 204.35
Percent Composition: C 88.16%, H 11.84%
Literature References: Sesquiterpenoid isomer of caryophyllene,
q.v. occurring in many essential oils, especially oil of hops
(Humulus lupulus L.
Moraceae) and leaves of
Lindera strychnifolia (F.) Will
Lauraceae. Occurs in nature as a mixture with b-humulene. Isolation of mixture: A. C. Chapman,
J. Chem. Soc. 67, 54, 780 (1895). Identity with a-caryophyllene: F. Sorm
et al., Collect. Czech. Chem. Commun. 14, 693, 699, 716 (1949). Structure: F. Sorm
et al., ibid. 19, 570 (1954). Stereochemistry: A. T. McPhail, G. A. Sim,
J. Chem. Soc. B 1966, 112. Synthesis: E. J. Corey, E. Hanamaka,
J. Am. Chem. Soc. 89, 2758 (1967). Stereoselective synthesis: Y. Kitagawa
et al., ibid. 99, 3864 (1977); E. J. Corey
et al., Tetrahedron Lett. 34, 3675 (1993). Chromatographic conversion to b-humulene: V. Benesova
et al., Collect. Czech. Chem. Commun. 26, 1832 (1961).
Reviews: F. Sorm in
Fortschr. Chem. Org. Naturst. 19, 1-32 (1961);
Rodd's Chemistry of Carbon Compounds vol. IIC, S. Coffey, Ed., (Elsevier, New York, 2nd ed., 1969) pp 282-283.
Properties: Liquid. bp5 106-107°.
nD30 1.5004, N. P. Damodaran, S. Dev,
Tetrahedron 24, 4113 (1968). Also reported as bp10 123°.
nD25 1.5015. d425 0.8865, R. P. Hildebrand
et al., Chem. Ind. (London) 1959, 489. NMR spectrum: S. Dev
et al., J. Am. Chem. Soc. 90, 1246 (1968).
Boiling point: bp5 106-107°; bp10 123°
Index of refraction: nD30 1.5004;
nD25 1.5015
Density: d425 0.8865
Derivative Type: Silver nitrate complex
Molecular Formula: C15H24.2AgNO3
Molecular Weight: 544.10
Percent Composition: C 33.11%, H 4.45%, Ag 39.65%, N 5.15%, O 17.64%
Properties: Crystals from aq ethanol, mp 175°.
Melting point: mp 175°
Derivative Type: b-Humulene
CAS Registry Number: 116-04-1
CAS Name: (
E,E)-1,4,4-Trimethyl-8-methylene-1,5-cycloundecadiene
Properties: Liquid.
nD20 1.5014. d420 0.8905.
Index of refraction: nD20 1.5014
Density: d420 0.8905