Title: Cefaclor
CAS Registry Number: 70356-03-5
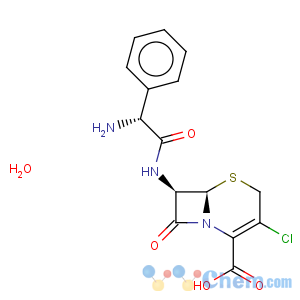
CAS Name: (6
R,7
R)-7-[[(2
R)-Aminophenylacetyl]amino]-3-chloro-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid monohydrate
Synonyms: 7-(D-2-amino-2-phenylacetamido)-3-chloro-3-cephem-4-carboxylic acid monohydrate; 3-chloro-7-D-(2-phenylglycinamido)-3-cephem-4-carboxylic acid monohydrate
Manufacturers' Codes: compd 99638
Trademarks: Alfacet (Galenika); Alfatil (Lilly); Ceclor (Lilly); Distaclor (Lilly); Panacef (Lilly); Panoral (Lilly)
Molecular Formula: C15H14ClN3O4S.H2O
Molecular Weight: 385.82
Percent Composition: C 46.70%, H 4.18%, Cl 9.19%, N 10.89%, O 20.73%, S 8.31%
Literature References: Semi-synthetic cephalosporin antibiotic, related to cephalexin,
q.v. Prepn: R. R. Chauvette,
DE 2408698 (1974 to Lilly),
C.A. 82, 4278n (1975);
US 3925372 (1975 to Lilly); R. R. Chauvette, P. A. Pennington,
J. Med. Chem. 18, 403 (1975).
In vitro studies: M. S. Silver
et al., Antimicrob. Agents Chemother. 12, 591 (1977); R. N. Jones,
J. Antibiot. 30, 753 (1977); B. R. Meyers, S. Z. Hirschman,
J. Clin. Pharmacol. 18, 85 (1978). Metabolism: N. G. Waterman, L. F. Sharfenberger,
Antimicrob. Agents Chemother. 14, 614 (1978). Human pharmacology: G. R. Hodges
et al., ibid. 454; A. Glynne
et al., J. Antimicrob. Chemother. 4, 343 (1978). Clinical studies: J. D. Nelson
et al., Am. J. Dis. Child. 132, 992 (1978); B. M. Gray
et al., Antimicrob. Agents Chemother. 13, 988 (1978). Comprehensive description: L. J. Lorenz,
Anal. Profiles Drug Subs. 9, 107-123 (1980).
Properties: Crystalline solid. uv max (pH 7 buffer): 265 nm (e 6800). Sol in water. Practically insol in methanol, chloroform, benzene. Solns are stable at pH 2.5-4.5.
Absorption maximum: uv max (pH 7 buffer): 265 nm (e 6800)
Therap-Cat: Antibacterial.
Keywords: Antibacterial (Antibiotics); ?Lactams; Cephalosporins.