Title: Spermine
CAS Registry Number: 71-44-3
CAS Name: N,N¢-Bis(3-aminopropyl)-1,4-butanediamine
Synonyms: N,N¢-bis(3-aminopropyl)tetramethylenediamine; gerontine; musculamine; neuridine
Molecular Formula: C10H26N4
Molecular Weight: 202.34
Percent Composition: C 59.36%, H 12.95%, N 27.69%
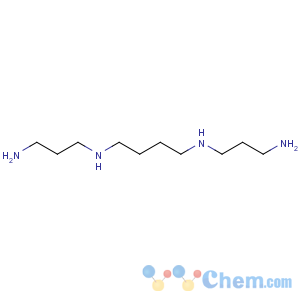
Line Formula: H2N(CH2)3NH(CH2)4NH(CH2)3NH2
Literature References: Biogenic polyamine formed from spermidine,
q.v., and occurring in almost all tissues. Essential for both normal and neoplastic tissue growth. First observed in human semen and described as the cryst phosphate salt by A. von Leeuwenhoek,
Philos. Trans. R. Soc. London 12, 1040 (1678). For a description of the history, occurrence, formation, and early prepns of spermine,
see Beilstein vol. IV, Suppl. 1, 704; M. Guggenheim,
Die biogenen Amine (S. Karger, Basel, 4th ed., 1951) 619 pp; H. Tabor
et al., Annu. Rev. Biochem. 30, 579-604 (1961). Prepn of spermine and its tetrahydrochloride: Israel
et al., J. Med. Chem. 7, 710 (1964). Role in cell growth processes: C. W. Tabor, H. Tabor,
Annu. Rev. Biochem. 45, 285 (1976); J. Janne
et al., Biochim. Biophys. Acta 473, 241 (1978). Modulation of calcium-dependent immune processes: T. C. Theoharides,
Life Sci. 27, 703 (1980). Biosynthesis in fungi: L. Stevens,
Med. Biol. 59, 308 (1981). HPLC study: C. E. Prussak, D. H. Russell,
J. Chromatogr. 229, 47 (1982). Use as a biochemical marker for malignant tumors: Y. Horn
et al., Cancer Res. 42, 3248 (1982). Metabolic study: A. E. Pegg
et al., Biochemistry 21, 5082 (1982). Review of role in cell proliferation and differentiation: O. Heby,
Differentiation 19, 1-20 (1981). Book:
Polyamines in Biology and Medicine D. R. Morris, L. J. Marton, Eds. (Dekker, New York, 1981) 512 pp.
Properties: Needles, mp 55-60°. Liq, bp0.5 141-142°. Strong base, absorbs carbon dioxide from air.
Keep well closed. Sol in water, lower alcohols, chloroform. Practically insol in ether, benzene, petr ether.
Melting point: mp 55-60°
Boiling point: bp0.5 141-142°
Derivative Type: Diphosphate hexahydrate
Synonyms: Spermine phosphate
Molecular Formula: C10H32N4O8P2.6H2O
Molecular Weight: 506.42
Percent Composition: C 23.72%, H 8.76%, N 11.06%, O 44.23%, P 12.23%
Properties: Cryst from water, mp 230-234° (dec). Soly in water: 0.037% at 20°; 1% at 100°. Insol in alc, ether and other organic solvents. Sol in dilute acids and alkali. Known as
Charcot-Neumann crystals, also found in spleen, blood, bone marrow in leukemia and secretions in asthma.
Melting point: mp 230-234° (dec)
Derivative Type: Tetrahydrochloride
Molecular Formula: C10H30Cl4N4
Molecular Weight: 348.18
Percent Composition: C 34.50%, H 8.68%, Cl 40.73%, N 16.09%
Properties: Cryst from ethanol, mp 312-314.5°.
Melting point: mp 312-314.5°
Use: As a tool in biochemical research.