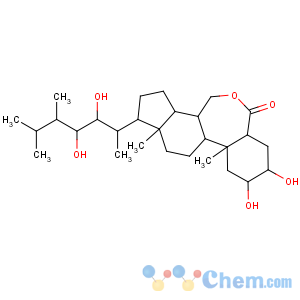
Title: Brassinolide
CAS Registry Number: 72962-43-7
CAS Name: (2a,3a,5a,22
R,23
R,24
S)-2,3,22,23-Tetrahydroxy-
B-homo-7-oxaergostan-6-one
Synonyms: 2a,3a,22,23-tetrahydroxy-24-methyl-B-homo-7-oxa-5a-cholestan-6-one
Molecular Formula: C28H48O6
Molecular Weight: 480.68
Percent Composition: C 69.96%, H 10.07%, O 19.97%
Literature References: Plant hormone; natural steroid containing a seven-membered B-ring lactone, that promotes both cell elongation and cell division. Over ten brassinosteroids have been isolated and characterized from sources such as pollen, seedling, leaf. Isoln, structure and activity of brassinolide from rape pollen,
Brassica napus L.: M. D. Grove
et al., Nature 281, 216 (1979). Stereoselective synthesis: S. Fung, J. B. Siddall,
J. Am. Chem. Soc. 102, 6580 (1980). Synthesis of two stereoisomers: M. J. Thompson
et al., J. Org. Chem. 44, 5002 (1979). Improved synthesis: T. Kametani
et al., J. Org. Chem. 53, 1982 (1988). Structure-activity relationship of brassinosteroids: S. Takatsuto
et al., Phytochemistry 22, 2437 (1983); interaction with cytokinin: C. Schlagnhaufer
et al., Physiol. Plant. 60, 347 (1984); bioassay: K. Wada
et al., Agric. Biol. Chem. 48, 719 (1984).
Properties: Crystals from methanol, mp 274-275°. [a]D27 +16°.
Melting point: mp 274-275°
Optical Rotation: [a]D27 +16°
Use: Plant growth regulator.