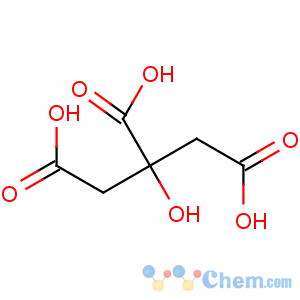
Title: Citric Acid
CAS Registry Number: 77-92-9
CAS Name: 2-Hydroxy-1,2,3-propanetricarboxylic acid
Synonyms: b-hydroxytricarballylic acid
Molecular Formula: C6H8O7
Molecular Weight: 192.12
Percent Composition: C 37.51%, H 4.20%, O 58.29%
Literature References: Widely distributed in plants and in animal tissues and fluids. Produced by mycological fermentation on an industrial scale using crude sugar solns, such as molasses and strains of
Aspergillus niger: See review by Von Loesecke,
Chem. Eng. News 23, 1952 (1945); Schweiger,
US 2970084 (1961 to Miles Labs.);
Faith, Keyes & Clark's Industrial Chemicals, F. A. Lowenheim, M. K. Moran, Eds. (Wiley-Interscience, New York, 4th ed., 1975) pp 275-279. Also extracted from citrus fruits (lemon juice contains 5 to 8%) and from pineapple waste.
Reviews: Wilson,
Chem. Metall. Eng. 29, 787 (1923); Browne,
Ind. Eng. Chem. 13, 81 (1921); Warneford, Hardy,
ibid. 17, 1283 (1925); E. F. Bouchard, E. G. Merritt in
Kirk-Othmer Encyclopedia of Chemical Technology vol. 6 (Wiley-Interscience, New York, 3rd ed., 1979) pp 150-179. Toxicity: C. M. Gruber, Jr., W. A. Halbeisen,
J. Pharmacol. Exp. Ther. 94, 65 (1948).
Properties: Anhydr form, mp 153°. Crystals are monoclinic holohedra and crystallize from hot concd aq soln. d 1.665. At 25°, pK1 3.128; pK2 4.761; pK3 6.396, Bates, Pinching,
J. Am. Chem. Soc. 71, 1274 (1949). Soly in water: 54.0% w/w at 10°; 59.2% at 20°; 64.3% at 30°; 68.6% at 40°; 70.9% at 50°; 73.5% at 60°; 76.2% at 70°; 78.8% at 80°; 81.4% at 90°; 84.0% at 100°. LD50 in mice, rats (mmol/kg): 5.0, 4.6 i.p. (Gruber, Halbeisen).
Melting point: mp 153°
pKa: pK1 3.128; pK2 4.761; pK3 6.396, Bates, Pinching,
J. Am. Chem. Soc. 71, 1274 (1949)
Density: d 1.665
Toxicity data: LD50 in mice, rats (mmol/kg): 5.0, 4.6 i.p. (Gruber, Halbeisen)
Derivative Type: Monohydrate
Properties: Orthorhombic crystals from cold aq solns. Pleasant, sour taste. d 1.542. Monohydrate crystals lose water of crystn in dry air or when heated at about 40 to 50°, slightly deliquescent in moist air. Softens at 75°. mp ~100°. pH of 0.1
N soln = 2.2. Densities of aqueous soln (15°/15°): 10% = 1.0392; 20% = 1.0805; 30% = 1.1244; 40% = 1.1709; 50% = 1.2204; 60% = 1.2738. Soly in g/100 g satd soln: ether 2.17; chloroform 0.007; amyl alcohol 15.43; amyl acetate 5.98; ethyl acetate 5.28. Soly at 19° in g/100 g solvent: methanol 197; propanol 62.8.
Melting point: mp ~100°
Density: d 1.542; Densities of aqueous soln (15°/15°): 10% = 1.0392; 20% = 1.0805; 30% = 1.1244; 40% = 1.1709; 50% = 1.2204; 60% = 1.2738
Derivative Type: Barium salt heptahydrate
Synonyms: Barium citrate
Molecular Formula: C12H10Ba3O14.7H2O
Molecular Weight: 916.29
Percent Composition: C 15.73%, H 2.64%, Ba 44.96%, O 36.67%
Properties: Powder. Loses all H2O at 150°. Sol in 1750 parts water; freely sol in dil HCl or HNO3; practically insol in alcohol.
Derivative Type: Ethyl ester
Synonyms: Ethyl citrate; triethyl citrate
Molecular Formula: C12H20O7
Molecular Weight: 276.28
Percent Composition: C 52.17%, H 7.30%, O 40.54%
Properties: Bitter, oily liq. d20 1.137. bp760 294°; bp1.0 127°. Viscosity at 25°: 35.2 cP. Pour pt ~10°.
nD20 1.4455. Soly: water ~6.9%; peanut oil 0.8%. Misc with alc, ether.
Boiling point: bp760 294°; bp1.0 127°
Index of refraction: nD20 1.4455
Density: d20 1.137
Derivative Type: Disodium salt
CAS Registry Number: 144-33-2
Synonyms: Disodium hydrogen citrate; sodium acid citrate
Trademarks: Alkacitron (PDH)
Molecular Formula: C6H6Na2O7
Molecular Weight: 236.09
Percent Composition: C 30.52%, H 2.56%, Na 19.48%, O 47.44%
Properties: White powder, saline taste. One gram of sesquihydrate dissolves in slightly less than 2 ml water; pH of a 3% w/v soln in water: 4.9 to 5.2. LD50 in mice, rats (mmol/kg): 7.5, 7.3 i.p. (Gruber, Halbeisen).
Toxicity data: LD50 in mice, rats (mmol/kg): 7.5, 7.3 i.p. (Gruber, Halbeisen)
Derivative Type: Trisodium salt
Literature References: See Sodium Citrate.
Use: Anticoagulant, generally in solution with glucose, to prevent the clotting of blood intended for transfusion. Acidulant in beverages, confectionery, effervescent salts, in pharmaceutical syrups, elixirs, in effervescent powders and tablets, to adjust the pH of foods and as synergistic antioxidant, in processing cheese. Used in beverages, jellies, jams, preserves and candy to provide tartness. In the manuf of alkyd resins; in esterified form as plasticizer, foam inhibitor. In the manuf of citric acid salts. As sequestering agent to remove trace metals. As mordant to brighten colors; in electroplating; in special inks; in analytical chemistry for determining citrate-soluble P2O5; as reagent for albumin, mucin, glucose, bile pigments.