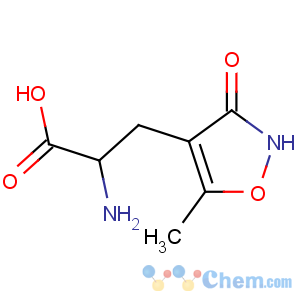
Title: AMPA
CAS Registry Number: 77521-29-0
CAS Name: a-Amino-2,3-dihydro-5-methyl-3-oxo-4-isoxazolepropanoic acid
Synonyms: a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
Molecular Formula: C7H10N2O4
Molecular Weight: 186.17
Percent Composition: C 45.16%, H 5.41%, N 15.05%, O 34.38%
Literature References: Synthetic excitatory amino acid that characterizes a specific subset of ionotropic glutamate receptors in the CNS, consequently known as AMPA-receptors. The activity resides primarily in the L-isomer. Prepn: J. J. Hansen, P. Krogsgaard-Larsen,
J. Chem. Soc. Perkin Trans. 1 1980, 1826; M. Begtrup, F. A. Slok,
Synthesis 1993, 861. Characterization of neuroexcitatory activity: P. Krogsgaard-Larsen
et al., Nature 284, 64 (1980). Resolution of enantiomers and stereospecific activity: J. J. Hansen
et al., J. Med. Chem. 26, 901 (1983). Review of AMPA receptors: K. Borges, R. Dingledine,
Prog. Brain Res. 116, 153-170 (1998); and potential for pharmacological intervention: G. J. Lees,
Drugs 59, 33-78 (2000).
Properties: Crystals from water as the monohydrate, mp 252° (dec).
Melting point: mp 252° (dec)
Derivative Type: L-AMPA
CAS Registry Number: 83643-88-3
Synonyms: S-AMPA
Properties: Crystals from water + ethanol as the hydrate, gradual decomp above ~200°. [a]D28 -21 ±2° (c = 0.19 in water).
Optical Rotation: [a]D28 -21 ±2° (c = 0.19 in water)