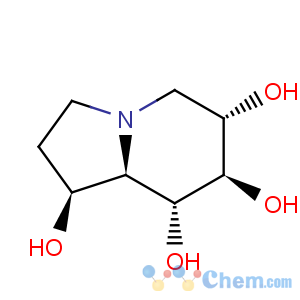
Title: Castanospermine
CAS Registry Number: 79831-76-8
CAS Name: (1
S,6
S,7
R,8
R,8a
R)-Octahydro-1,6,7,8-indolizinetetrol
Synonyms: 1,6,7,8-tetrahydroxyoctahydroindolizine; (1
S,6
S,7
R,8
R,8a
R)-1,6,7,8-tetrahydroxyindolizidine
Molecular Formula: C8H15NO4
Molecular Weight: 189.21
Percent Composition: C 50.78%, H 7.99%, N 7.40%, O 33.82%
Literature References: Polyhydroxy alkaloid isolated from the seeds of the Australian leguminous tree,
Castanospermum australe. Inhibits enzymatic glycoside hydrolysis. Isoln of the naturally occurring (+)-form: L. D. Hohenschutz
et al., Phytochemistry 20, 811 (1981). Total synthesis and absolute configuration: R. C. Bernotas, B. Ganem,
Tetrahedron Lett. 25, 165 (1984). Alternate synthesis: H. Hamana
et al., J. Org. Chem. 52, 5492 (1987). Inhibition of a- and b-glucosidases: R. Saul
et al., Arch. Biochem. Biophys. 221, 593 (1983);
eidem, ibid. 230, 668 (1984). Insect antifeedant activity: D. L. Dreyer
et al., J. Chem. Ecol. 11, 1045 (1985). Inhibition of HIV infectivity
in vitro: B. D. Walker
et al., Proc. Natl. Acad. Sci. USA 84, 8120 (1987); R. A. Gruters
et al., Nature 330, 74 (1987).
Properties: Crystals from aq ethanol, mp 212-215° (dec). [a]D25 +79.7° (c = 0.93 in water). pK 6.09.
Melting point: mp 212-215° (dec)
pKa: pK 6.09
Optical Rotation: [a]D25 +79.7° (c = 0.93 in water)