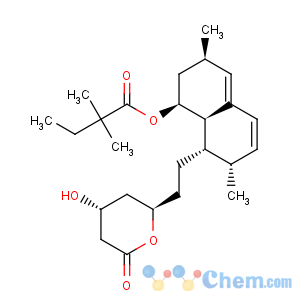
Title: Simvastatin
CAS Registry Number: 79902-63-9
CAS Name: 2,2-Dimethylbutanoic acid (1
S,3
R,7
S,8
S,8a
R)-1,2,3,7,8,8a-hexahydro-3,7-dimethyl-8-[2-[(2
R,4
R)-tetrahydro-4-hydroxy-6-oxo-2
H-pyran-2-yl]ethyl]-1-naphthalenyl ester
Synonyms: 2,2-dimethylbutyric acid 8-ester with (4
R,6
R)-6-[2-[(1
S,2
S,6
R,8
S,8a
R)-1,2,6,7,8,8a-hexahydro-8-hydroxy-2,6-dimethyl-1-naphthyl]ethyl]tetrahydro-4-hydroxy-2
H-pyran-2-one; synvinolin
Manufacturers' Codes: MK-733
Trademarks: Denan (Boehringer, Ing.); Liponorm (Gentili); Lodalès (Sanofi-Synthelabo); Simovil (Merck & Co.); Sinvacor (Merck & Co.); Sivastin (Sigma-Tau); Zocor (Merck & Co.); Zocord (Merck & Co.)
Molecular Formula: C25H38O5
Molecular Weight: 418.57
Percent Composition: C 71.74%, H 9.15%, O 19.11%
Literature References: HMG-CoA reductase inhibitor; synthetic analog of lovastatin,
q.v. Prepn: A. K. Willard
et al., EP 33538; W. F. Hoffman
et al., US 4444784 (1981, 1984 both to Merck & Co.); W. F. Hoffman
et al., J. Med. Chem. 29, 849 (1986). Comprehensive description: D. K. Ellison
et al., Anal. Profiles Drug Subs. Excip. 22, 359-388 (1993). Effect on survival in CHD: Scandinavian Simvastatin Survival Study Group,
Lancet 344, 1383 (1994). Clinical trial in primary hypercholesterolemia: L. Ose
et al., Clin. Cardiol. 23, 39 (2000); in mixed hyperlipidemia: E. Stein
et al., Am. J. Cardiol. 86, 406 (2000).
Properties: Crystals from
n-butyl chloride + hexane, mp 135-138°. Soly (mg/ml): chloroform 610; DMSO 540; methanol 200; ethanol 160;
n-hexane 0.15; 0.1
M HCl 0.06; polyethylene glycol-400 70; propylene glycol 30; 0.1
M NaOH 70; water 0.03. uv max (acetonitrile): 231, 238, 247 nm (A1%1 cm 516, 604, 408). [a]D25 +292° (c = 0.5% in acetonitrile).
Melting point: mp 135-138°
Optical Rotation: [a]D25 +292° (c = 0.5% in acetonitrile)
Absorption maximum: uv max (acetonitrile): 231, 238, 247 nm (A1%1 cm 516, 604, 408)
Therap-Cat: Antilipemic.
Keywords: Antilipemic; HMG CoA Reductase Inhibitors; HMG CoA Reductase Inhibitor.