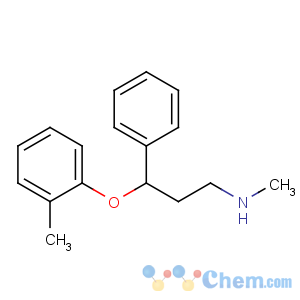
Title: Atomoxetine
CAS Registry Number: 83015-26-3
CAS Name: (g
R)-
N-Methyl-g-(2-methylphenoxy)benzenepropanamine
Synonyms: (-)-
N-methyl-3-(
o-tolyloxy)-3-phenylpropylamine; tomoxetine
Molecular Formula: C17H21NO
Molecular Weight: 255.35
Percent Composition: C 79.96%, H 8.29%, N 5.49%, O 6.27%
Literature References: Norepinephrine reuptake inhibitor. Prepn (stereochem unspec): B. B. Molloy, K. K. Schmiegel,
DE 2500110;
eidem,
US 4314081 (1975, 1982 both to Lilly). Prepn of (
R)-form: B. J. Foster, E. R. Lavagnino,
EP 52492 (1982 to Lilly); Y. Gao, K. B. Sharpless,
J. Org. Chem. 53, 4081 (1988). Clinical pharmacokinetics: N. A. Farid
et al., J. Clin. Pharmacol. 25, 296 (1985). Binding study: D. R. Gehlert
et al., J. Neurochem. 64, 2792 (1995). Biotransformation by human liver microsomes: B. J. Ring
et al., Drug Metab. Dispos. 30, 319 (2002). Evaluation of abuse potential: S. H. Heil
et al., Drug Alcohol Depend. 67, 149 (2002). Clinical trial in pediatric ADHD: C. J. Kratochvil
et al., J. Am. Acad. Child Adolesc. Psychiatry 41, 776 (2002); and comorbid tic disorders: A. J. Allen
et al., Neurology 65, 1941 (2005). Review of clinical pharmacokinetics: J.-M. Sauer
et al., Clin. Pharmacokinet. 44, 571-590 (2005).
Derivative Type: Hydrochloride
CAS Registry Number: 82248-59-7
Manufacturers' Codes: LY 139603
Trademarks: Strattera (Lilly)
Molecular Formula: C17H21NO.HCl
Molecular Weight: 291.82
Percent Composition: C 69.97%, H 7.60%, N 4.80%, O 5.48%, Cl 12.15%
Properties: Crystals, mp 166-168° (Foster, Lavagnino). [a]D25 -38.01°; [a]36525 -177.26° (c = 1 in methanol). Also reported as white solid from acetonitrile, mp 162-164° (Gao, Sharpless). [a]D23 -41.37° (c = 1.02 in methanol); [a]D25 -40.3° (c = 0.94 in ethanol). Soly in water: 27.8 mg/ml. pKa 10.13.
Melting point: mp 166-168°; mp 162-164° (Gao, Sharpless)
pKa: pKa 10.13
Optical Rotation: [a]D25 -38.01°; [a]36525 -177.26° (c = 1 in methanol); [a]D23 -41.37° (c = 1.02 in methanol); [a]D25 -40.3° (c = 0.94 in ethanol)
Therap-Cat: In treatment of attention deficit hyperactivity disorder (ADHD).