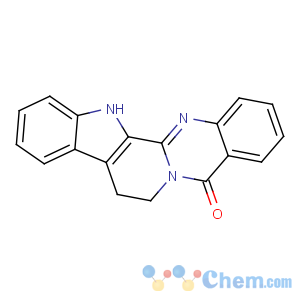
Title: Rutecarpine
CAS Registry Number: 84-26-4
CAS Name: 8,13-Dihydroindolo[2¢,3¢:3,4]pyrido[2,1-
b]quinazolin-5(7
H)-one
Synonyms: rutaecarpine
Molecular Formula: C18H13N3O
Molecular Weight: 287.32
Percent Composition: C 75.24%, H 4.56%, N 14.62%, O 5.57%
Literature References: From fruit of
Evodia rutaecarpa Hook. & Thoms. and
Hortia arborea Engl.,
Rutaceae: Asahina, Kashiwaki,
J. Pharm. Soc. Jpn. 1915, 1293; Pachter
et al., J. Am. Chem. Soc. 82, 5187 (1960). Structure: Y. Asahina,
J. Pharm. Soc. Jpn. 1924, 1. Synthesis: Y. Asahina
et al., J. Chem. Soc. 1927, 1708; T. Kametani
et al., J. Am. Chem. Soc. 99, 2306 (1977);
eidem, Chem. Pharm. Bull. 26, 1922 (1978); H. M?hrle
et al., Arch. Pharm. 313, 990 (1980); J. Bergman, S. Bergman,
Heterocycles 16, 347 (1981). Simple synthesis: J. K?k?si
et al., Tetrahedron Lett. 22, 4861 (1981). Synthesis under physiological conditions: Sch?pf,
Angew. Chem. 50, 779, 797 (1937). Biosynthesis: M. Yamazaki
et al., Tetrahedron Lett. 1966, 3221;
1967, 3317. Mass spec.: J. Tamas
et al., Acta Chim. Acad. Sci. Hung. 89, 85 (1976).
Properties: Needles from ethyl acetate, mp 259.5-260°. uv max (ethanol): 278, 290, 332, 345, 364 nm (log e 3.83, 3.88, 4.49, 4.54, 4.44). Sol in alc, benzene, chloroform, ether. Practically insol in water.
Melting point: mp 259.5-260°
Absorption maximum: uv max (ethanol): 278, 290, 332, 345, 364 nm (log e 3.83, 3.88, 4.49, 4.54, 4.44)