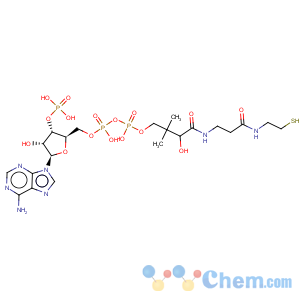
Title: Coenzyme A
CAS Registry Number: 85-61-0
Synonyms: CoA
Molecular Formula: C21H36N7O16P3S
Molecular Weight: 767.53
Percent Composition: C 32.86%, H 4.73%, N 12.77%, O 33.35%, P 12.11%, S 4.18%
Literature References: An essential cofactor in enzymatic acetyl transfer reactions. Synthesized in cells from pantothenate, ATP and cysteine. Found ubiquitously in mammalian cells. Isoln from animal sources: Lipmann
et al., J. Biol. Chem. 167, 869 (1947);
186, 235 (1950). Many microorganisms contain large amounts of the coenzyme. Isoln from
Streptomyces fradiae: Kaplan, Lipmann,
ibid. 174, 37 (1948). Purifications: De Vries
et al., J. Am. Chem. Soc. 72, 4838 (1950); Gregory
et al., ibid. 74, 854 (1952). Structure: Baddiley
et al., Nature 171, 76 (1953). Total synthesis: Moffatt, Khorana,
J. Am. Chem. Soc. 81, 1265 (1959);
83, 663 (1961); Shimizu
et al., Chem. Pharm. Bull. 13, 1142 (1965). Reviews: Lipmann,
Bacteriol. Rev. 17, 1-16 (1953); Baddiley,
Adv. Enzymol. 16, 1 (1955); Jaenicke, Lynen in
The Enzymes vol. 3, P. D. Boyer
et al., Eds. (Academic Press, New York, 2nd ed., 1960) pp 3-103. Review of metabolism: J. D. Robishaw, J. R. Neely,
Am. J. Physiol. 248, E1-E9 (1985); of clinical evaluations in hyperlipoproteinemia: A. Perin, G. Fraticelli,
Int. J. Tissue React. 13, 111-114 (1991); of biochemical role in cellular toxicity: E. P. Brass,
Chem. Biol. Interact. 90, 203-214 (1994).
Properties: White powder; characteristic thiol odor. May be dried
in vacuo over phosphorus pentoxide at 34°. uv max: 259.5 nm (e 16800). Fairly strong acid. pK 9.6 (thiol); pK 6.4 (secondary phosphate); pK 4.0 (adenine NH3+). Soluble in water. Practically insol in ethanol, ether, acetone. Pure dry coenzyme is best stored in evacuated ampuls at room temp. Readily oxidized by air to the catalytically inactive disulfide.
pKa: pK 9.6 (thiol); pK 6.4 (secondary phosphate); pK 4.0 (adenine NH3+)
Absorption maximum: uv max: 259.5 nm (e 16800)