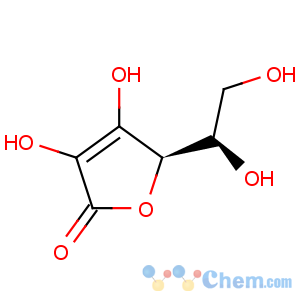
Title: Isoascorbic Acid
CAS Registry Number: 89-65-6
CAS Name: D-
erythro-Hex-2-enonic acid g-lactone
Synonyms: D-araboascorbic acid; erythorbic acid; isovitamin C; saccharosonic acid; glucosaccharonic acid; D-
erythro-3-ketohexonic acid lactone; D-
erythro-3-oxohexonic acid lactone; erycorbin
Trademarks: Mercate "5"; Neo-Cebicure
Molecular Formula: C6H8O6
Molecular Weight: 176.12
Percent Composition: C 40.92%, H 4.58%, O 54.51%
Literature References: Epimer of L-ascorbic acid. Has one-twentieth of the vitamin C activity of L-ascorbic acid. Prepd by treating methyl 2-keto-D-gluconate with sodium methoxide: Maurer, Schiedt,
Ber. 66, 1054 (1933);
67, 1239 (1934); Ohle
et al., ibid. 67, 324 (1934); Baird
et al., J. Chem. Soc. 1934, 63; Reichstein
et al., Helv. Chim. Acta 17, 516 (1934). Synthesis from sucrose: Heimann, Reiff,
Pharm. Zentralhalle 93, 97 (1954). Production by
Penicillium spp: Takahashi
et al., Nature 188, 411 (1960);
US 3052609 (1962 to Sankyo);
Bull. Agric. Chem. Soc. Jpn. 24, 533 (1960),
C.A. 55, 1788 (1961). Conformation: Matsui
et al., Agric. Biol. Chem. 27, 185 (1963).
Properties: Shiny granular crystals from water or dioxane. Dec 174°. [a]D16.5 -17° (c = 1.8 in 0.01
N HCl); [a]D20 -16.6° (H2O). Soluble in water, alc, pyridine. Moderately sol in acetone. Slightly sol in glycerol.
Optical Rotation: [a]D16.5 -17° (c = 1.8 in 0.01
N HCl); [a]D20 -16.6° (H2O)
Derivative Type: Sodium salt
Synonyms: Sodium erythorbate
Trademarks: Mercate "20"; Neo-Cebitate
Properties: Crystals, sol in water. pH of aq solns of the sodium salt between 5 and 6. A 10% soln, made from commercial grade, may have a pH of 7.2 to 7.9. The free acid is more sol in water (40 g/100 ml H2O) than the sodium salt (16 g/100 ml H2O).
Use: Antioxidant and antimicrobial agent for foods: Kadin, Osadca,
J. Agric. Food Chem. 7, 358 (1959).