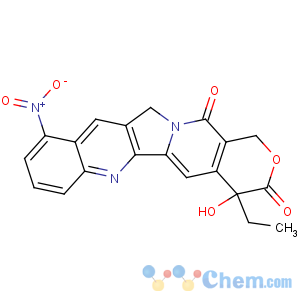
Title: Rubitecan
CAS Registry Number: 91421-42-0
CAS Name: (4
S)-4-Ethyl-4-hydroxy-10-nitro-1
H-pyrano[3¢,4¢:6,7]indolizino[1,2-
b]quinoline-3,14(4
H,12
H)-dione
Synonyms: 9-nitrocamptothecin; 9-nitro-(20
S)-camptothecin; 9-NC
Trademarks: Orathecin (SuperGen)
Molecular Formula: C20H15N3O6
Molecular Weight: 393.35
Percent Composition: C 61.07%, H 3.84%, N 10.68%, O 24.40%
Literature References: Semisynthetic camptothecin which inhibits DNA topoisomerase I. Prodrug of 9-aminocamptothecin, (9-AC),
q.v. Prepn:
JP Kokai 59 51288 (1984 to Yakult Honsha),
C.A. 101, 91322 (1984); M. C. Wani
et al., J. Med. Chem. 29, 2358 (1986); and activity: S. Sawada
et al., Chem. Pharm. Bull. 39, 3183 (1991). Conversion to 9-AC by human cells: P. Pantazis
et al., Eur. J. Haematol. 53, 246 (1994);
eidem, ibid. 55, 211 (1995). Clinical trial in pancreatic cancer: J. S. Stehlin
et al., Int. J. Oncol. 14, 821 (1999).
Review: C. F. Verschraegen,
Curr. Opin. Oncol. Endocr. Metab. Invest. Drugs 1, 184-190 (1999).
Properties: Yellow amorphous powder from CH3OH-CHCl3 (13:87), mp 182-186°. [a]D23 +27° (c = 0.2 in CH3OH-CHCl3, 1:4).
Melting point: mp 182-186°
Optical Rotation: [a]D23 +27° (c = 0.2 in CH3OH-CHCl3, 1:4)
Therap-Cat: Antineoplastic.
Keywords: Antineoplastic; Alkaloids/Natural Products; Camptothecin Derivatives.