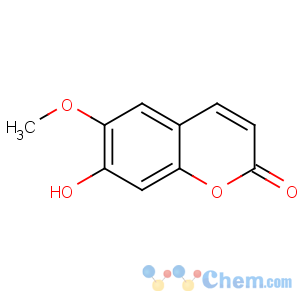
Title: Scopoletin
CAS Registry Number: 92-61-5
CAS Name: 7-Hydroxy-6-methoxy-2
H-1-benzopyran-2-one
Synonyms: 7-hydroxy-6-methoxycoumarin; 6-methoxyumbelliferone; b-methylesculetin; chrysatropic acid; gelseminic acid
Molecular Formula: C10H8O4
Molecular Weight: 192.17
Percent Composition: C 62.50%, H 4.20%, O 33.30%
Literature References: The aglucone of scopolin. Occurs in root of
Scopolia japonica Maxim.,
Scopolia carniolica Jacq.,
Atropa belladonna L.,
Solanaceae, Convolvulus scammonia L.,
Convolvulaceae. Isoln: Eykman,
Ber. 17 III, 442 (1884). Synthesis: Crosby,
J. Org. Chem. 26, 1215 (1961); Desai, Desai,
J. Indian Chem. Soc. 40, 456 (1963).
Properties: Needles or prisms from chloroform or acetic acid, mp 204°. uv max: 230, 254, 260, 298, 346 nm (log e 4.11, 3.68, 3.63, 3.68, 4.07), Ballantyne
et al., Tetrahedron 27, 871 (1971). Slightly sol in water or cold alcohol; sol in hot alcohol or hot glacial acetic acid; moderately sol in chloroform. Practically insol in benzene. Its alcoholic soln has a blue fluorescence. Reduces Fehling's soln.
Melting point: mp 204°
Absorption maximum: uv max: 230, 254, 260, 298, 346 nm (log e 4.11, 3.68, 3.63, 3.68, 4.07), Ballantyne
et al., Tetrahedron 27, 871 (1971)