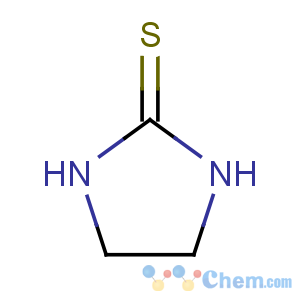
Title: Ethylene Thiourea
CAS Registry Number: 96-45-7
CAS Name: 2-Imidazolidinethione
Synonyms: imidazoline-2-thiol; 2-mercaptoimidazoline; ETU
Trademarks: Akrochem ETU-22 (Akron Chem.); NA-22 (DuPont); Robac 22 (Robinson Bros.); Sanceller 22 (Sanshin); Vulkacit NPV/C (Mobay)
Molecular Formula: C3H6N2S
Molecular Weight: 102.16
Percent Composition: C 35.27%, H 5.92%, N 27.42%, S 31.39%
Literature References: Degradation product of ethylenebisdithiocarbamate fungicides such as mancozeb, maneb, zineb,
q.q.v. Prepn: C. F. H. Allen
et al., Org. Synth. coll. vol. III, 394 (1955); G. Matolcsy,
Ber. 101, 522 (1968). Identification as decomposition product: R A. Ludwig
et al., Can. J. Bot. 32, 48 (1954); G. Czegledi-Janko,
J. Chromatogr. 31, 89 (1967). Determn in various foods by GLC: W. H. Newsome,
J. Agric. Food Chem. 20, 967 (1972); by GLC, HPLC: J. H. Onley
et al., J. Assoc. Off. Anal. Chem. 60, 1105 (1977); J. H. Onley,
ibid. 1111. Review of determn methods: P. Bottomley
et al., Residue Rev. 95, 45-89 (1985). Metabolism in mice: K. Savolainen, H. Pyysalo,
J. Agric. Food Chem. 27, 1177 (1979); in cats and rats: F. Iverson,
Toxicol. Appl. Pharmacol. 52, 16 (1980). Aquatic toxicity data: C. J. Van Leeuwen,
Aquat. Toxicol. 7, 145 (1985). Goitrogenic activity: S. L. Graham, W. H. Hansen,
Bull. Environ. Contam. Toxicol. 7, 19 (1972); D. M. Smith,
Br. J. Ind. Med. 41, 362 (1984). Use in rubber industry as crosslinking agent: J. T. Oetzel,
Rubber World 172, 55 (1975); C. Hepburn, M. S. Mahdi,
Kautsch. Gummi Kunstst. 39, 629 (1986). Review of toxicology: L. Fishbein,
J. Toxicol. Environ. Health 1, 713-756 (1976); of genotoxicity studies: K. L. Dearfield,
Mutat. Res. 317, 111-132 (1994).
Properties: Needles, prisms from alc or amyl alc. mp 203-204°. Soly in 100 ml water: 2 g at 30°, 9 g at 60°, 44 g at 90°. Moderately sol in methanol, ethanol, ethylene glycol, and pyridine. Insol in acetone, ether, chloroform, benzene, ligroin. LD50 orally in rats: 1832 mg/kg (Graham, Hansen).
Melting point: mp 203-204°
Toxicity data: LD50 orally in rats: 1832 mg/kg (Graham, Hansen)
CAUTION: Potential symptom of overexposure is eye irritation.
See NIOSH Pocket Guide to Chemical Hazards (DHHS/NIOSH 97-140, 1997) p 138. This substance is reasonably anticipated to be a human carcinogen:
Report on Carcinogens, Eleventh Edition (PB2005-104914, 2004) p III-122.
Use: Accelerator in synthetic rubber production.