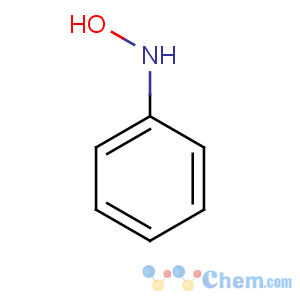
Title: Phenylhydroxylamine
CAS Registry Number: 100-65-2
CAS Name: N-Hydroxybenzenamine
Synonyms: b-phenylhydroxylamine;
N-phenylhydroxylamine
Molecular Formula: C6H7NO
Molecular Weight: 109.13
Percent Composition: C 66.04%, H 6.47%, N 12.83%, O 14.66%
Line Formula: C6H5NHOH
Literature References: Prepd by zinc reduction of nitrobenzene in ammonium chloride soln: Kamm,
Org. Synth. 4, 57 (1925).
Properties: Needles from satd NaCl soln. mp 82°.
Deteriorates on storage and should be used promptly. Sol in 50 parts cold, in 10 parts hot water. Freely sol in alcohol, ether, carbon disulfide, chloroform, hot benzene, dil mineral acids, acetic acid. Slightly sol in petr ether. The oxalate is more stable.
Melting point: mp 82°
Use: Manufacture of cupferron.