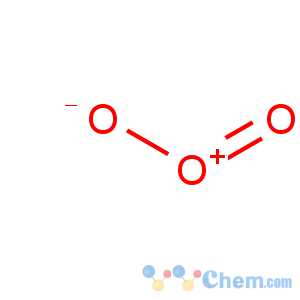
Title: Ozone
CAS Registry Number: 10028-15-6
Synonyms: Triatomic oxygen
Molecular Formula: O3
Molecular Weight: 48.00
Percent Composition: O 100.00%
Literature References: Found in the atm in varying proportions (about 0.05 ppm at sea level), since it is produced continuously in the outer layers of the atm by the action of solar uv radiation on the oxygen of the air. So-called sterilizing lamps operate on the same principle. In the laboratory ozone is prepd by passing dry air between two plate electrodes connected to an alternating current source of several thousand volts. The reaction is reversible, and after a little ozone has been produced it is dec at the same rate as it is generated. Obtained in pure form by cooling ozonized air to -180° when it separates as a dark blue liquid.
See also C. E. Thorp,
Bibliography of Ozone Technology (Armour Res. Found., Chicago). Lab prepn:
Org. Synth. coll. vol. III, 673 (1955). Conference proceedings:
Adv. Chem. Ser. 21, entitled "Ozone Chemistry and Technology," H. A. Leedy, Ed. (ACS, Washington D.C., 1959) 465 pp.
Review: C. Nebel in
Kirk-Othmer Encyclopedia of Chemical Technology vol. 16 (Wiley-Interscience, New York, 3rd ed., 1981) pp 683-713.
Properties: Bluish, explosive gas or blue liquid. Pleasant, characteristic odor in concns of less than 2 ppm. Irritating and injurious in higher concns. Powerful oxidizing agent. d0 (gas): 2.144 g/l; d-195.4 (liq) 1.614 g/ml. mp -193°. bp -111.9°. Critical temp -12.1°. Critical press. 53.8 atm. Heat of formation 34.4 kcal/mole at 25°. Intense absorption band beginning at about 290 nm. Unstable. Solutions contg ozone explode on warming. Prepn of ozone solns in liq oxygen: Cook,
US 3008902 (1961 to Union Carbide). Although the stability of ozone in aq solns decreases as alkalinity rises, this effect is reversed at high concns. For example, the half life of ozone is 2 min in 1
N NaOH; it is increased to 83 hrs in 20
N NaOH: Heidt, Landi,
J. Chem. Phys. 41, 176 (1964).
Melting point: mp -193°
Boiling point: bp -111.9°
Density: d0 (gas): 2.144 g/l; d-195.4 (liq) 1.614 g/ml
CAUTION: Potential symptoms of overexposure are irritation of eyes and mucous membranes; pulmonary edema; chronic respiratory disease.
See NIOSH Pocket Guide to Chemical Hazards (DHHS/NIOSH 97-140, 1997) p 238.
Use: As disinfectant for air and water by virtue of its oxidizing power. For bleaching waxes, textiles, oils. In organic syntheses. Forms ozonides which are sometimes useful oxidizing compds.