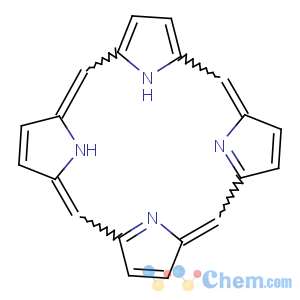
Title: Porphine
CAS Registry Number: 101-60-0
CAS Name: 21
H,23
H-Porphine
Synonyms: porphin
Molecular Formula: C20H14N4
Molecular Weight: 310.35
Percent Composition: C 77.40%, H 4.55%, N 18.05%
Literature References: Parent substance of the
porphyrins, a group of compounds found in all living matter which are the basis of respiratory pigments in animals and plants. In porphyrins, side chains are substituted for the hydrogens in the porphine pyrrole rings.
Chlorins are dihydroporphyrins.
See also: chlorophyll, hemoglobin, vitamin B12, hematin. Prepn of porphine: Fischer, Gleim,
Ann. 521, 157 (1935); Rothemund,
J. Am. Chem. Soc. 58, 625 (1936); Krol,
J. Org. Chem. 24, 2065 (1959). Study of porphyrin analogs: C. L. Honeybourne
et al., Tetrahedron 36, 1833 (1980). Review of biosyntheses of porphyrins and chlorins: A. R. Battersby, E. McDonald in
Porphyrins Metalloporphyrins, K. M. Smith, Ed. (Elsevier, New York, 1975) pp 61-122. Review of porphyrin syntheses: R. P. Evstigneeva,
Pure Appl. Chem. 53, 1129-1140 (1981). Comprehensive seven volume treatise:
The Porphyrins, D. Dolphin, Ed. (Academic Press, New York, 1978).
Properties: Dark red, shiny leaflets from chloroform-methanol. Darkens at 360° but does not melt. The absorption bands are those characteristic of the substituted porphyrins, details in Fischer-Orth,
Die Chemie des Pyrrols vol. II, 1, 175 (1937). Sol in pyridine, dioxane, and phenol; slightly sol in chloroform, bromoform, glacial acetic acid. Almost insol in acetone, methanol, ether. HCl number: 1.7 (Fischer); 3.3 (Rothemund).
Derivative Type: Iron salt
Molecular Formula: C20H12N4.FeCl
Molecular Weight: 399.63
Percent Composition: C 60.11%, H 3.03%, N 14.02%, Fe 13.97%, Cl 8.87%
Properties: Brown cubes from ether.
Derivative Type: Magnesium salt
Molecular Formula: C20H12MgN4
Molecular Weight: 332.64
Percent Composition: C 72.21%, H 3.64%, Mg 7.31%, N 16.84%
Properties: Red needles.
Derivative Type: Copper salt
Molecular Formula: C20H12N4Cu
Molecular Weight: 371.88
Percent Composition: C 64.59%, H 3.25%, N 15.07%, Cu 17.09%
Properties: Brown needles.