Title: Aluminum Nitrate
CAS Registry Number: 13473-90-0
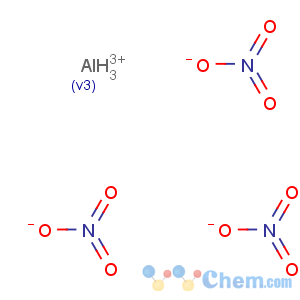
Molecular Formula: AlN3O9
Molecular Weight: 213.00
Percent Composition: Al 12.67%, N 19.73%, O 67.60%
Line Formula: Al(NO3)3
Literature References: Occurs in several states of hydration of which the nonahydrate is the most stable. Prepn:
Gmelins, Aluminum (8th ed.)
35B, p 149-152 (1934). Toxicity data: Smyth
et al., Am. Ind. Hyg. Assoc. J. 30, 470 (1969).
Derivative Type: Nonahydrate
Properties: Deliquesc crystals; mp 73°; dec at 135°. Very sol in water, alc; very slightly sol in acetone. Almost insol in ethyl acetate and pyridine. The aq soln is acid.
Keep well closed. LD50 orally in rats: 4.28 g/kg (Smyth).
Melting point: mp 73°; dec at 135°
Toxicity data: LD50 orally in rats: 4.28 g/kg (Smyth)
Use: Tanning leather; antiperspirant; corrosion inhibitor; extraction of uranium; nitrating agent.