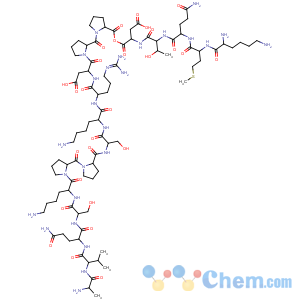
References of L-Aspartic acid,L-alanyl-L-valyl-L-glutaminyl-L-seryl-L-lysyl-L-prolyl-L-prolyl-L-seryl-L-lysyl-L-arginyl-L-a-aspartyl-L-prolyl-L-prolyl-L-lysyl-L-methionyl-L-glutaminyl-L-threonyl-
Title: Systemin
CAS Registry Number: 137181-56-7
CAS Name: L-Alanyl-L-valyl-L-glutaminyl-L-seryl-L-lysyl-L-prolyl-L-prolyl-L-seryl-L-lysyl-L-argininyl-L-a-aspartyl-L-prolyl-L-prolyl-L-lysyl-L-methionyl-L-glutaminyl-L-threonyl-L-aspartic acid
Literature References: First known polypeptide signal in plants. Systemically induces plant defenses in response to wounding of leaves by insects or pathogens. Proline-rich palindromic structure. Identification of activity: C. A. Ryan,
Plant Physiol. 54, 328 (1974). Isolation from tomato leaves and characterization: G. Pearce
et al., Science 253, 895 (1991). Purification by capillary electrophoresis: P. Mucha
et al., J. Chromatogr. A 734, 410 (1996). Proton NMR assignments: D. J. Russell
et al., J. Protein Chem. 11, 265 (1992). Structure-activity: T. Meindl
et al., Plant Cell 10, 1561 (1998). Tertiary structure and mechanism of action: T. Specht
et al., Plants Proteins Eur. Crops 1998, 41.
Review: C. A. Ryan, G. Pearce,
Annu. Rev. Cell Dev. Biol. 14, 1-17 (1998).
Properties: Insol in lipid solvents.
Use: Wound hormone in plants.