References of 2-piperidin-1-ylethyl 3-methyl-4-oxo-2-phenylchromene-8-carboxylate
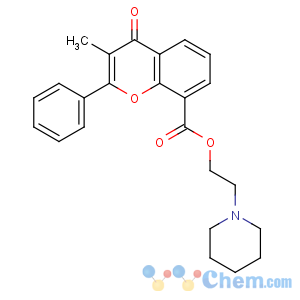
Title: Flavoxate
CAS Registry Number: 15301-69-6
CAS Name: 3-Methyl-4-oxo-2-phenyl-4
H-1-benzopyran-8-carboxylic acid 2-(1-piperidinyl)ethyl ester
Synonyms: 3-methylflavone-8-carboxylic acid b-piperidinoethyl ester; 2-piperidinoethyl 3-methyl-4-oxo-2-phenyl-4
H-1-benzopyran-8-carboxylate; 2-piperidinoethyl 3-methylflavone-8-carboxylate
Molecular Formula: C24H25NO4
Molecular Weight: 391.46
Percent Composition: C 73.64%, H 6.44%, N 3.58%, O 16.35%
Literature References: Smooth muscle relaxant. Prepn of the hydrochloride: P. Da Re
et al., J. Med. Pharm. Chem. 2, 263 (1960); P. Da Re,
US 2921070 and
US 3350411 (1960 to Recordati and 1967 to Seceph). Pharmacological studies: I. Setnikar
et al., J. Pharmacol. Exp. Ther. 130, 356 (1960). Mechanism of action: P. Cazzulani
et al., Arch. Int. Pharmacodyn. 274, 189 (1985). Pharmacokinetics in rats: I. Setnikar
et al., Arzneim.-Forsch. 25, 1916 (1975); in man: M. Bertoli
et al., Pharmacol. Res. Commun. 8, 417 (1976).
In vivo activity: C. Pietra, P. Cazzulani,
Farmaco Ed. Prat. 41, 267 (1986). Determn in tissues: A. Cova, I. Setnikar,
Arzneim.-Forsch. 25, 1707 (1975). Clinical evaluations: D. V. Bradley, R. J. Cazort,
J. Clin. Pharmacol. 10, 65 (1970); A. Zanollo, F. Catanzaro,
Urol. Int. 35, 176 (1980). Clinical comparison with phenazopyridine: S. Gould,
Urology 5, 612 (1975). Short review: R. Ruffmann, A. Sartini,
Drugs Exp. Clin. Res. 13, 57 (1987).
Properties: Crystals, pK 7.3. Soly in water at 37°: 0.001% (w/v). Sol in ethanol and chloroform. LD50 in rats (mg/kg): 1110 orally; 20.8 i.v. (Setnikar, 1975).
pKa: pK 7.3
Toxicity data: LD50 in rats (mg/kg): 1110 orally; 20.8 i.v. (Setnikar, 1975)
Derivative Type: Hydrochloride
CAS Registry Number: 3717-88-2
Manufacturers' Codes: DW-61; Rec-7-0040
Trademarks: Bladderon (Nippon Shinyaku); Genurin (Recordati); Patricin (Kaken); Spasuret (Asche); Urispas (SKB)
Molecular Formula: C24H25NO4.HCl
Molecular Weight: 427.92
Percent Composition: C 67.36%, H 6.12%, N 3.27%, O 14.96%, Cl 8.28%
Properties: Crystals from ethanol + ether, mp 232-234°. LD50 i.v. in rats: 27.4 mg/kg (Cazzulani).
Melting point: mp 232-234°
Toxicity data: LD50 i.v. in rats: 27.4 mg/kg (Cazzulani)
Derivative Type: Succinate
CAS Registry Number: 28782-19-6
Molecular Formula: C24H25NO4.C4H6O4
Molecular Weight: 509.55
Percent Composition: C 66.00%, H 6.13%, N 2.75%, O 25.12%
Properties: Soly in water at 37°: 33.7% (w/v).
Therap-Cat: Antispasmodic; in treatment of urinary incontinence.
Keywords: Antispasmodic; Antimuscarinic.